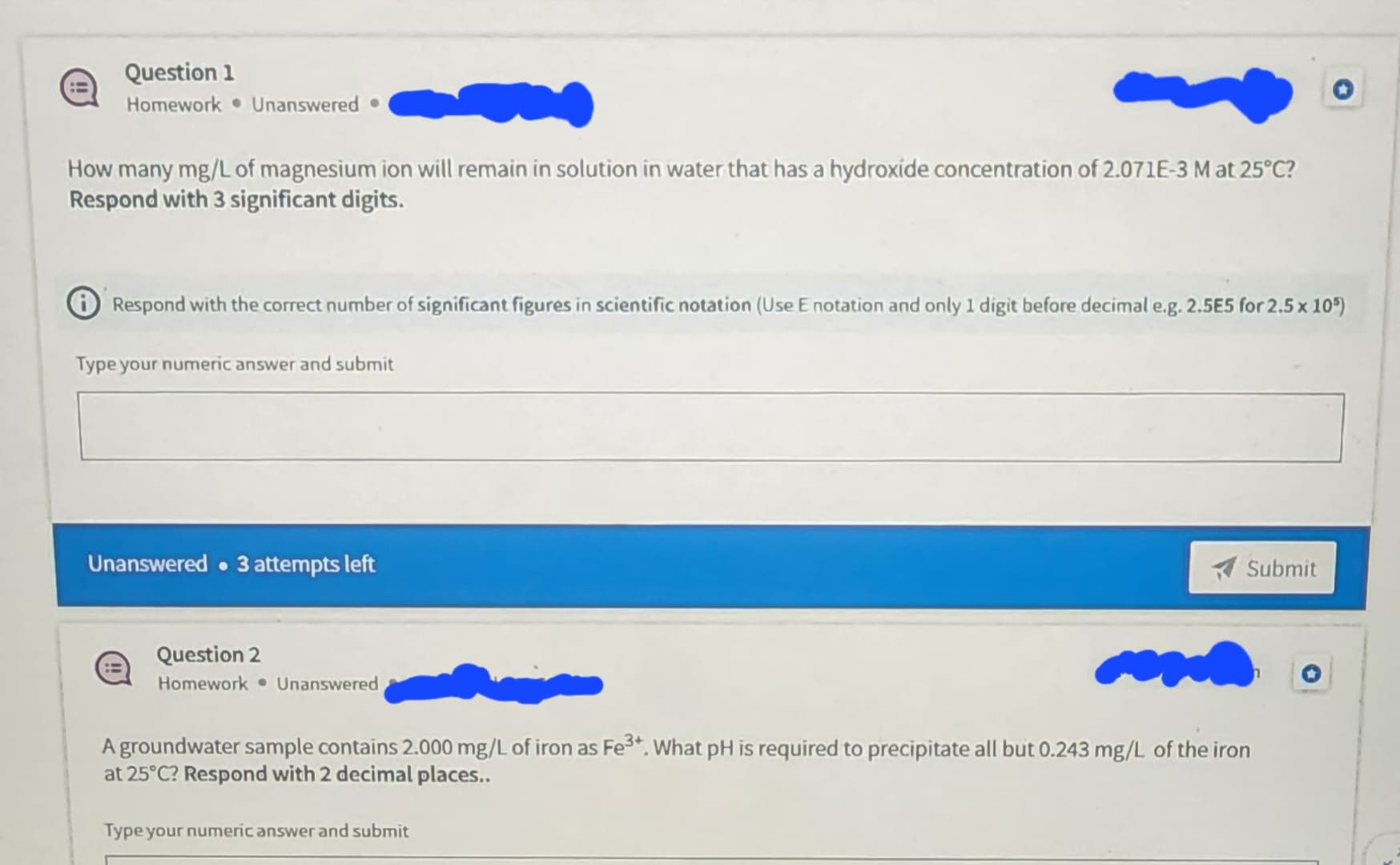
How many mg/L of magnesium ion will remain in solution in water that has a hydroxide concentration of 2.071E-3 M at 25°C? Respond with 3 significant digits. Respond with the correct number of significant figures in scientific notation (Use E notation and only 1 digit before decimal e.g. 2.5E5 for 2.5 x 105)
How many mg/L of magnesium ion will remain in solution in water that has a hydroxide concentration of 2.071E-3 M at 25°C? Respond with 3 significant digits. Respond with the correct number of significant figures in scientific notation (Use E notation and only 1 digit before decimal e.g. 2.5E5 for 2.5 x 105)
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
Transcribed Image Text:~
How many mg/L of magnesium ion will remain in solution in water that has a hydroxide concentration of 2.071E-3 M at 25°C?
Respond with 3 significant digits.
Question 1
Homework Unanswered.
Respond with the correct number of significant figures in scientific notation (Use E notation and only 1 digit before decimal e.g. 2.5E5 for 2.5 x 105)
Type your numeric answer and submit
Unanswered 3 attempts left
Question 2
Homework Unanswered
Submit
M
A groundwater sample contains 2.000 mg/L of iron as Fe³+. What pH is required to precipitate all but 0.243 mg/L of the iron
at 25°C? Respond with 2 decimal places..
Type your numeric answer and submit
n
![Question 3
Homework Unanswered
+
Determine the pH that must be achieved to produce a concentration of 0.243 mg/L of copper if the starting concentration is 2.00
mg/L. Respond with 2 decimal places.
Type your numeric answer and submit
Unanswered 3 attempts left
Question 4
Homework Unanswered
Submit
Calculate the pH of a water at 25°C that contains 0.579 mg/L of carbonic acid. Assume that [H+] = [HCO3-] at equilibrium and
neglect the dissociation of water. Round your answer to 3 decimal places.
Type your numeric answer and submit](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fbfe9d25a-4775-4c82-aed5-03aabe9f56d3%2F5635d73a-d1ce-430c-8f28-b1aa9720e5ae%2Ffjau57e.jpeg&w=3840&q=75)
Transcribed Image Text:Question 3
Homework Unanswered
+
Determine the pH that must be achieved to produce a concentration of 0.243 mg/L of copper if the starting concentration is 2.00
mg/L. Respond with 2 decimal places.
Type your numeric answer and submit
Unanswered 3 attempts left
Question 4
Homework Unanswered
Submit
Calculate the pH of a water at 25°C that contains 0.579 mg/L of carbonic acid. Assume that [H+] = [HCO3-] at equilibrium and
neglect the dissociation of water. Round your answer to 3 decimal places.
Type your numeric answer and submit
Expert Solution
Step 1
We’ll answer the first question since the exact one wasn’t specified. Please submit a new question specifying the one you’d like answered.
1.
Given that-
Hydroxide concentration [OH-]=2.07 x 10-3 M=0.00207 M
Temperature, T= 250C
Here, the solution has 0.00207 moles of hydroxide ion in 1 liter of solution.
The reaction of magnesium ion with hydroxide ion is as follows-
The solubility product is-
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The