Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter3: Chemical Reactions
Section: Chapter Questions
Problem 70QRT
Related questions
Question
How do you do these questions? I have 3 problems linked below.
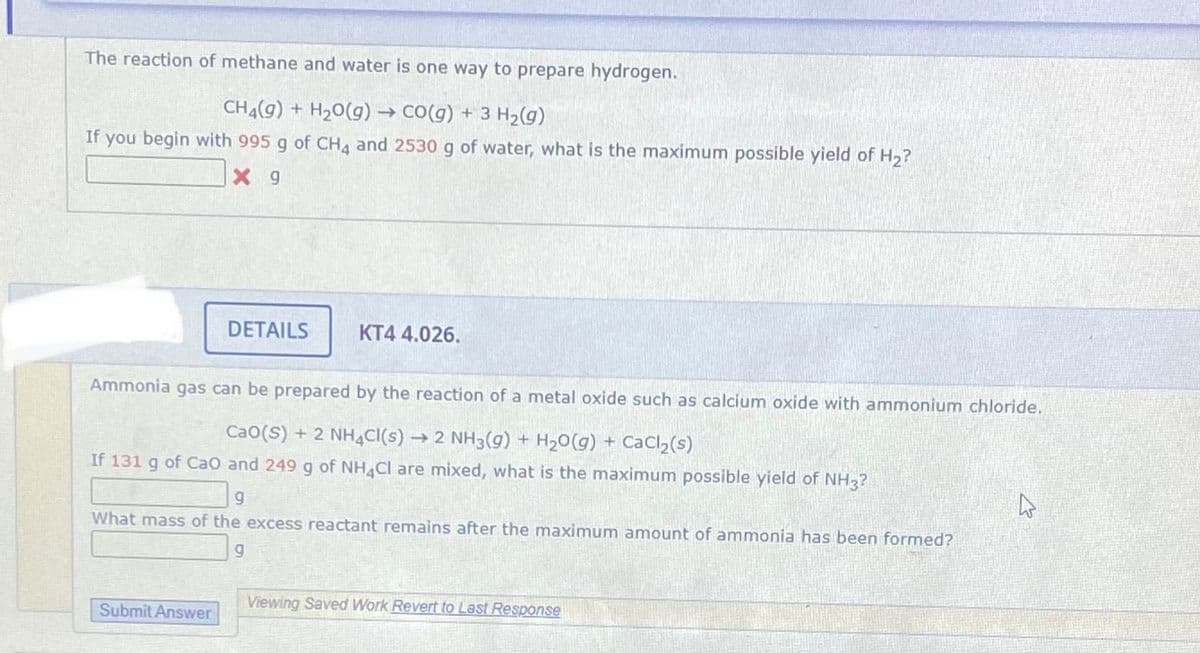
Transcribed Image Text:The reaction of methane and water is one way to prepare hydrogen.
CH4(g) + H20(g) → CO(g) + 3 H2(g)
If
you begin with 995 g of CHA and 2530 g of water, what is the maximum possible yield of H2?
X 9
DETAILS
KT4 4.026.
Ammonia gas can be prepared by the reaction of a metal oxide such as calcium oxide with ammonium chloride.
Cao(S) + 2 NH4CI(s)
→ 2 NH3(g) + H20(g) + CaCl,(s)
If 131 g of Cao and 249 g of NH Cl are mixed, what is the maximum possible yield of NH3?
What mass of the excess reactant remains after the maximum amount of ammonia has been formed?
5.
Viewing Saved Work Revert to Last Response
Submit Answer
Transcribed Image Text:Clas: X
A Pres x
Flipc X
Q Lear x
UnV X
O Cop X
it Stat X
G engl x
W Stoic X
G Amn x
O NAN X
A webassign.net/web/Student/Assignment-Responses/last?dep=26448471#question166272_0
Apps My Schedule A Sign In
A Classes
S Home Schoology Edpuzzle s Pearson SuccessNet
m Course: Honors Ge..
A Research Links - Da.
O Home | Naviance St...
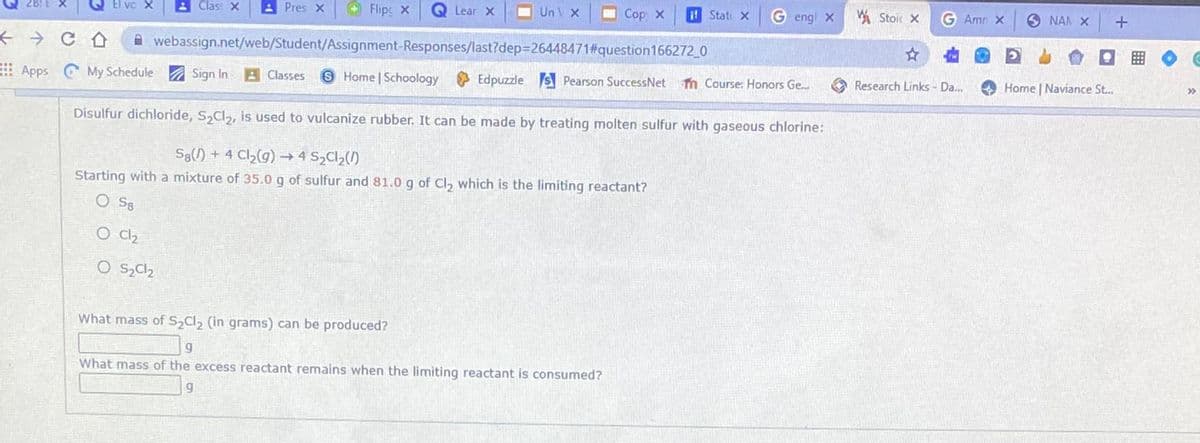
Disulfur dichloride, S,Cl,, is used to vulcanize rubber. It can be made by treating molten sulfur with gaseous chlorine:
S8() + 4 Cl2(g) → 4 S2C1,(1)
Starting with a mixture of 35.0 g of sulfur and 81.0 g of Cl, which is the limiting reactant?
O S8
O C2
What mass of S Cl, (in grams) can be produced?
What mass of the excess reactant remains when the limiting reactant is consumed?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning