Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter8: Gases
Section: Chapter Questions
Problem 155CP: Methane (CH4) gas flows into a combustion chamber at a rate of 200. L/min at 1.50 atm and ambient...
Related questions
Question
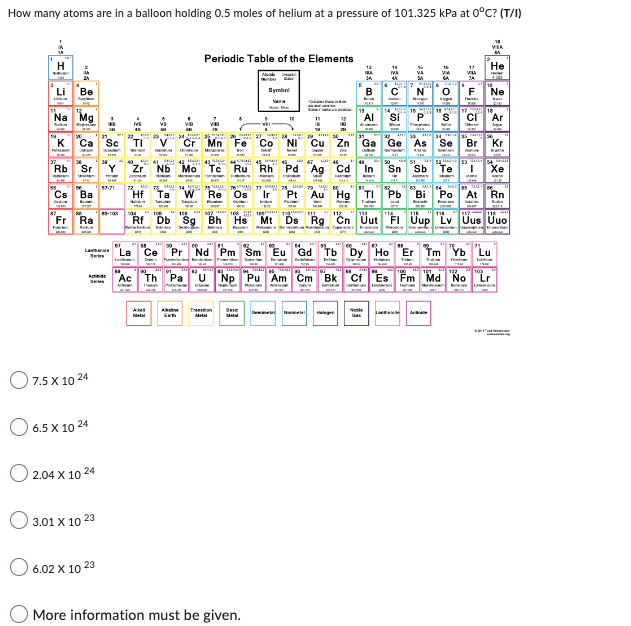
Transcribed Image Text:How many atoms are in a balloon holding 0.5 moles of helium at a pressure of 101.325 kPa at 0°C? (T/I)
H
Li
Na Mg
Be
Rb
Cs Ba
Ra
H
7.5 X 1024
6.5 X 1024
2.04 X 1024
3.01 X 10 23
6.02 X 10 23
TI
Periodic Table of the Elements
Mn
A
TH
Mak
42 43 44 45 45
Zr Nb Mo Tc Ru Rh Pd
P
76 77
Hf Ta W Re Os Ir
Symbol
Fe Co Ni
Basic
10
More information must be given.
75
Pt
Cu
Ag
S
Na
45
Cd
13
B
14
MA
Al Si
Ga
74
N
15.
Se
LABAN
Te
17
VILA
89-108 104 106
108
Rf Db
b Sg Bh Hs Mt Ds Rg Cn Uut F Uup Lv Uus Quo
VIA
La "Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr
He
FNe
Br Kr
Au Hg Tl Pb Bi Po At Rn
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning