Chapter3: Mass Spectrometry: Part One: Basic Theory, Instrumentation And Sampling Techniques
Section: Chapter Questions
Problem 6P
Related questions
Question
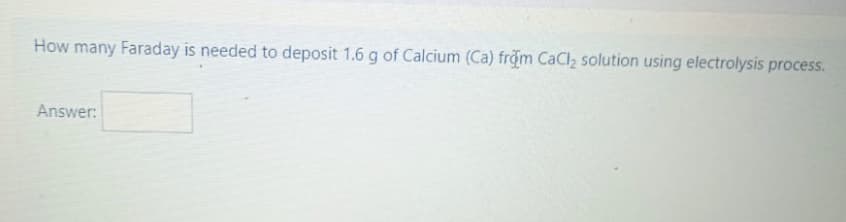
Transcribed Image Text:How many Faraday is needed to deposit 1.6 g of Calcium (Ca) from CaCl, solution using electrolysis process.
Answer:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax


University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax