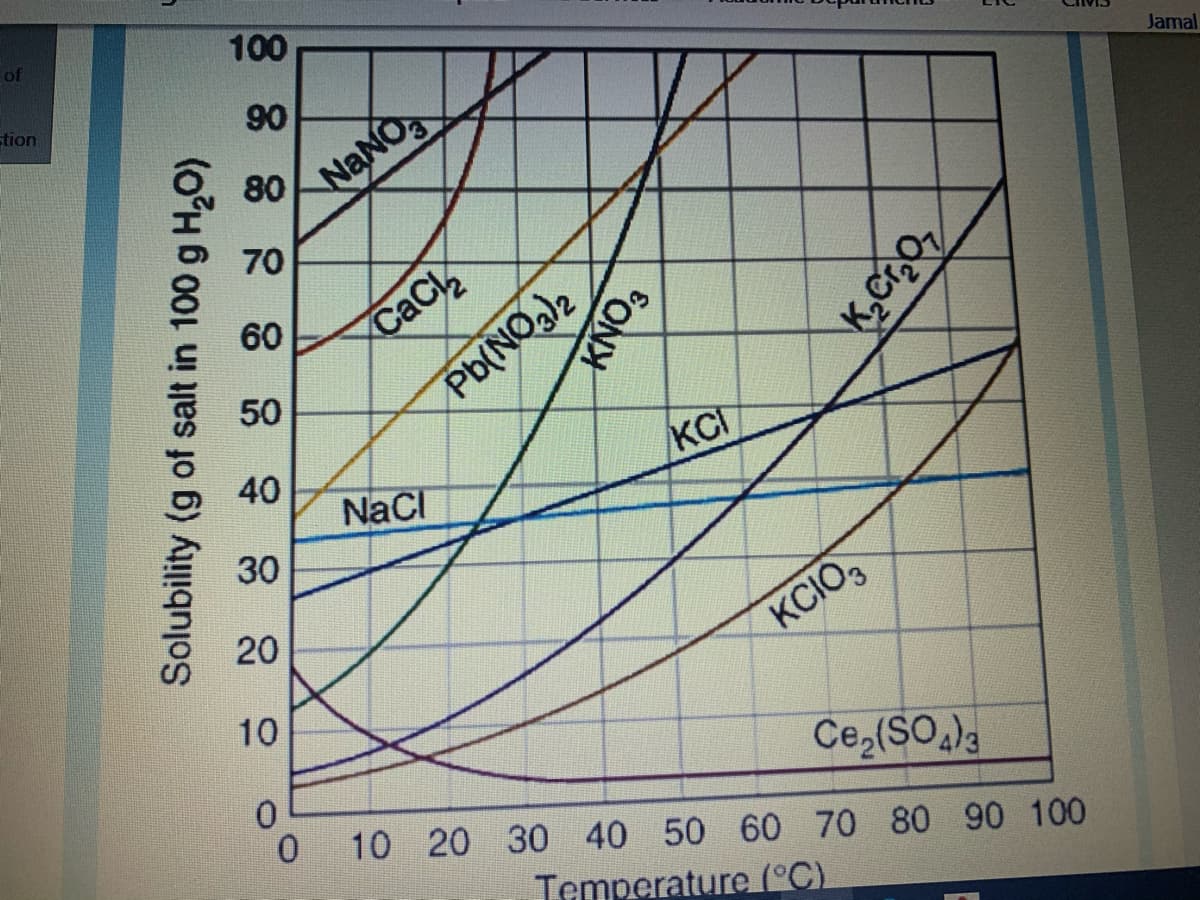
How many grams of NaNO3 will dissolve in 100 gram of water at Choose.. 30 °C? How many grams of KCI will dissolve in 50 gram of water at 50 °C ? 30 gram A saturated solution of KCIO3 prepared in 100 gram of water at 94 °C is cooled to 55 °C. How much KCIO3 will precipitate out of solution? Choose... Choose... In an aqueous solution of KCIO3, solute is
How many grams of NaNO3 will dissolve in 100 gram of water at Choose.. 30 °C? How many grams of KCI will dissolve in 50 gram of water at 50 °C ? 30 gram A saturated solution of KCIO3 prepared in 100 gram of water at 94 °C is cooled to 55 °C. How much KCIO3 will precipitate out of solution? Choose... Choose... In an aqueous solution of KCIO3, solute is
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter10: Solutions
Section: Chapter Questions
Problem 61QAP: A sucrose (C12H22O11) solution that is 45.0% sucrose by mass has a density of 1.203 g/mL at 25C....
Related questions
Question

Transcribed Image Text:How many grams of NaNO; will dissolve in 100 gram of water at
30 °C?
Choose.
How many grams of KCI will dissolve in 50 gram of water at 50 °C ?
30 gram
A saturated solution of KCIO3 prepared in 100 gram of water at 94 °C
is cooled to 55 °C. How much KCIO3 will precipitate out of solution?
Choose...
Choose...
In an aqueous solution of KCIO3, solute is
Transcribed Image Text:100
of
stion
90
NANO
80
Jamal
70
60
CaC
50
Pb(NOg)2
40
NaCI
KCI
30
20
KCIO
10
0.
Ce,(SO3
10 20 30 40 50 60 70 80 90 100
Temperature (°C)
Solubility (g of salt in 100 g H,O)
SONY
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning