How many millilitrs of CO gas would be produced by the complete reaction of 2.40 g of C solid at STP according to the following reaction? Remember 1 mol of an ideal gas has a volume of 22.4 Lat STP. C(s) + H,O(g) → CO(g) + H,(g)
How many millilitrs of CO gas would be produced by the complete reaction of 2.40 g of C solid at STP according to the following reaction? Remember 1 mol of an ideal gas has a volume of 22.4 Lat STP. C(s) + H,O(g) → CO(g) + H,(g)
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter9: Gases
Section: Chapter Questions
Problem 77E: Ethanol, C2H5OH, is produced industrially from ethylene, C2H4, by the following sequence of...
Related questions
Question
Fill in the blanks Correctly.
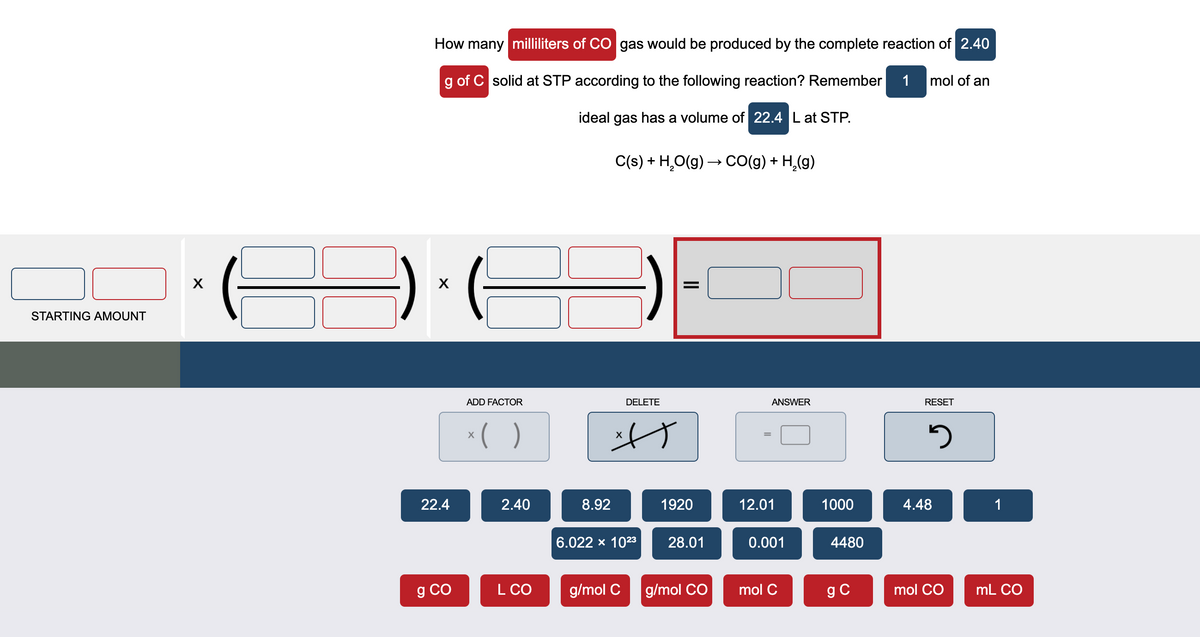
Transcribed Image Text:How many milliliters of CO gas would be produced by the complete reaction of 2.40
g of C solid at STP according to the following reaction? Remember
1 mol of an
ideal gas has a volume of 22.4 L at STP.
C(s) + H,O(g) → CO(g) + H,(g)
STARTING AMOUNT
ADD FACTOR
DELETE
ANSWER
RESET
22.4
2.40
8.92
1920
12.01
1000
4.48
1
6.022 x 1023
28.01
0.001
4480
g CO
L CO
g/mol C
g/mol CO
mol C
g C
mol CO
mL CO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning