How many mL of HCI, 0.1 M, will you need to prepare 0.700 L of HCL, 0.025 M? Student A and Student B were analyzing the initial set up of the first problem. Step 1: Identify and label knowns and unknown •Known Unknown M (initial) = 0.1 M V(initial) = ? mL M (final ) = 0.025 M V (final) = 0.700 L %3! Step 2: Write the base equation for dilution M() x V() = M (f) x V (f) Step 3: Solve for the unknown and substitute the values. V() = (0.025 M)(0.700 L) = 0.175 L 0.1 M Student B said, "The dilution equation works, because the number of particles of solute that is transferred does not change during the dilution." Student A replied, "That is correct. You only add water to dilute the solution, so the number of solute particles does not change." You consider Student A and Student B to be correct. O True O False
How many mL of HCI, 0.1 M, will you need to prepare 0.700 L of HCL, 0.025 M? Student A and Student B were analyzing the initial set up of the first problem. Step 1: Identify and label knowns and unknown •Known Unknown M (initial) = 0.1 M V(initial) = ? mL M (final ) = 0.025 M V (final) = 0.700 L %3! Step 2: Write the base equation for dilution M() x V() = M (f) x V (f) Step 3: Solve for the unknown and substitute the values. V() = (0.025 M)(0.700 L) = 0.175 L 0.1 M Student B said, "The dilution equation works, because the number of particles of solute that is transferred does not change during the dilution." Student A replied, "That is correct. You only add water to dilute the solution, so the number of solute particles does not change." You consider Student A and Student B to be correct. O True O False
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter14: Chemical Equilibrium
Section: Chapter Questions
Problem 94AP
Related questions
Question
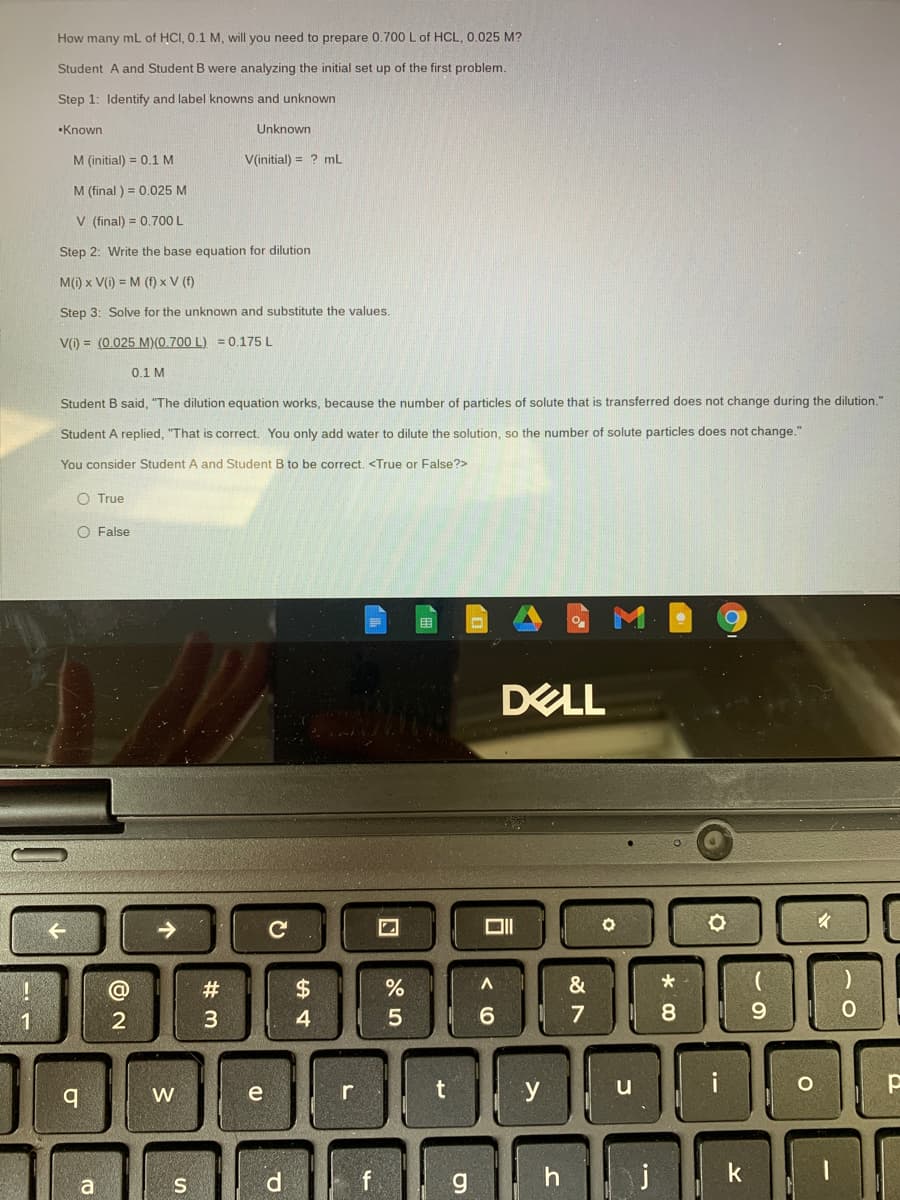
Transcribed Image Text:How many mL of HCI, 0.1 M, will you need to prepare 0.700 L of HCL, 0.025 M?
Student A and Student
were analyzing the initial set up of the first problem.
Step 1: Identify and label knowns and unknown
•Known
Unknown
M (initial) = 0.1 M
V(initial) = ? mL
M (final ) = 0.025 M
V (final) = 0.700 L
Step 2: Write the base equation for dilution
M(1) x V(i) = M (f) x V (f)
Step 3: Solve for the unknown and substitute the values.
V(i) = (0.025 M)(0.700 L) = 0.175 L
0.1 M
Student B said, "The dilution equation works, because the number of particles of solute that is transferred does not change during the dilution."
Student A replied, "That is correct. You only add water to dilute the solution, so the number of solute particles does not change."
You consider Student A and Student B to be correct. <True or False?>
O True
O False
DELL
->
$4
&
2
4
6.
7
8
9.
y u
i
W
e
r
f
h | j
k
# M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning



EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT