Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter10: Gases And Their Properties
Section10.3: The Ideal Gas Law
Problem 10.8CYU: A 0.105-g sample of a gaseous compound has a pressure of 561 mm Hg in a volume of 125 mL at 23.0 C....
Related questions
Question
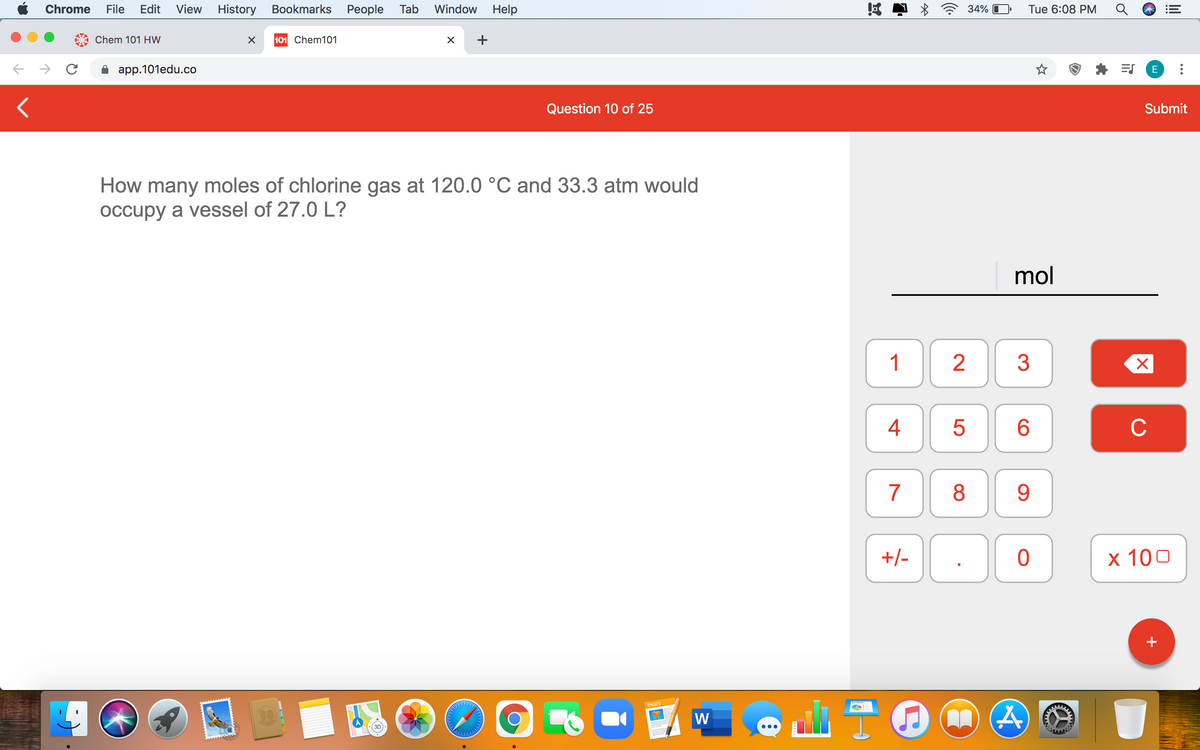
How many moles of chlorine gas at 120.0 °C and 33.3 atm would occupy a vessel of 27.0 L?
Transcribed Image Text:Chrome
File
Edit
View
History Bookmarks People Tab
Window
Help
34%
Tue 6:08 PM
Chem 101 HW
101 Chem101
+
app.101edu.co
E
Question 10 of 25
Submit
How many moles of chlorine gas at 120.0 °C and 33.3 atm would
occupy a vessel of 27.0 L?
mol
1
3
6.
C
7
8
9
+/-
x 100
+
PAGES
W
4+
Expert Solution
Step 1
Ideal gases are those gases which follow ideal gas equation. The relation between pressure, volume, number of moles, ideal gas constant and temperature is represented by this equation. Write the expression of Ideal gas equation.
where P is the pressure of the gas in atm, V is the volume of the gas in L, n is the number of moles, R is the ideal gas constant and T is the temperature of the gas.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning