How much energy (in J) is required to evaporate 25% of 4g of water currently at 100 °c? The answer (in fundamental S unit) is (type the numeric value only, dont use scientific notation)
How much energy (in J) is required to evaporate 25% of 4g of water currently at 100 °c? The answer (in fundamental S unit) is (type the numeric value only, dont use scientific notation)
Classical Dynamics of Particles and Systems
5th Edition
ISBN:9780534408961
Author:Stephen T. Thornton, Jerry B. Marion
Publisher:Stephen T. Thornton, Jerry B. Marion
Chapter4: Nonlinear Oscillations And Chaos
Section: Chapter Questions
Problem 4.13P
Related questions
Question
How much energy (in J) is required to evaporate 25% of 4g of water currently at 100 oC?
The answer (in fundamental SI unit) is ___________ (type the numeric value only, dont use scientific notation)
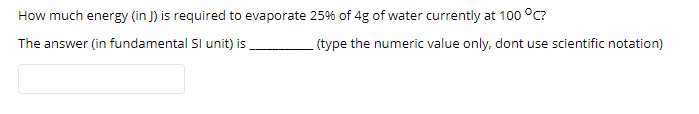
Transcribed Image Text:How much energy (in J) is required to evaporate 25% of 4g of water currently at 100 °C?
The answer (in fundamental Sl unit) is
(type the numeric value only, dont use scientific notation)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Classical Dynamics of Particles and Systems
Physics
ISBN:
9780534408961
Author:
Stephen T. Thornton, Jerry B. Marion
Publisher:
Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Classical Dynamics of Particles and Systems
Physics
ISBN:
9780534408961
Author:
Stephen T. Thornton, Jerry B. Marion
Publisher:
Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning