How to apply Raoult's law to real solutions Consider mixing a liquid with a vapor pressure of 100 torr with an equimolar amount of a liquid with a vapor pressure of 200 torr. The resulting solution would be predicted to have a vapor pressure of 150 torr if it behaved ideally. If, however, the interactions between the different components are not similar we can see positive or negative deviations from the calculated vapor pressure. An actual vapor pressure greater than that predicted by Raoult's law is said to be a positive deviation and an actual vapor pressure lower than that predicted by Raoult's law is a negative deviation. Part A Imagine a solution of two liquids in which the molecules interact less favorably than they do in the individual liquids. Will this solution deviate positively from, deviate negatively from, or ideally follow Raoult's law? • View Available Hint(s) It will deviate positively. O t will deviate negatively. O It will be an ideal solution.
How to apply Raoult's law to real solutions Consider mixing a liquid with a vapor pressure of 100 torr with an equimolar amount of a liquid with a vapor pressure of 200 torr. The resulting solution would be predicted to have a vapor pressure of 150 torr if it behaved ideally. If, however, the interactions between the different components are not similar we can see positive or negative deviations from the calculated vapor pressure. An actual vapor pressure greater than that predicted by Raoult's law is said to be a positive deviation and an actual vapor pressure lower than that predicted by Raoult's law is a negative deviation. Part A Imagine a solution of two liquids in which the molecules interact less favorably than they do in the individual liquids. Will this solution deviate positively from, deviate negatively from, or ideally follow Raoult's law? • View Available Hint(s) It will deviate positively. O t will deviate negatively. O It will be an ideal solution.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter12: Solutions
Section: Chapter Questions
Problem 12.88QE
Related questions
Question
100%
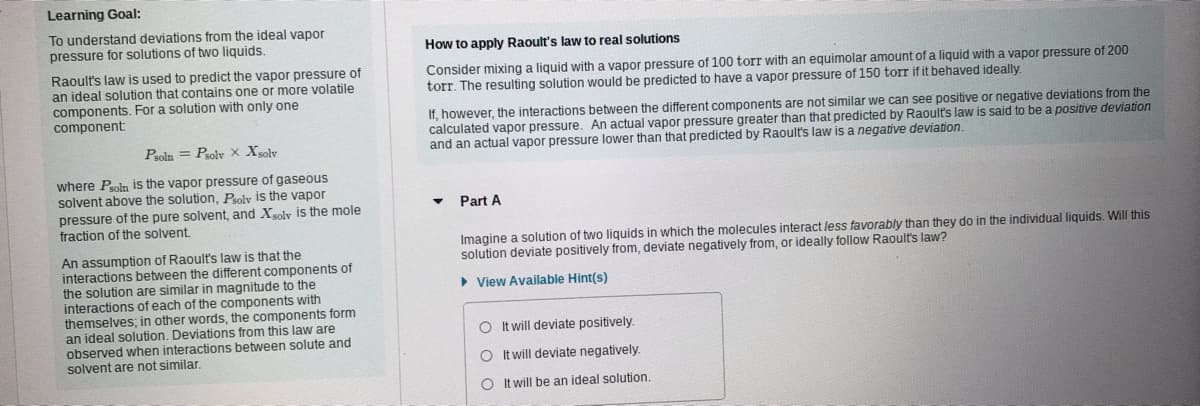
Transcribed Image Text:Learning Goal:
To understand deviations from the ideal vapor
pressure for solutions of two liquids.
How to apply Raoult's law to real solutions
Raoult's law is used to predict the vapor pressure of
an ideal solution that contains one or more volatile
components. For a solution with only one
component
Consider mixing a liquid with a vapor pressure of 100 torr with an equimolar amount of a liquid with a vapor pressure of 200
torr. The resulting solution would be predicted to have a vapor pressure of 150 torr if it behaved ideally.
If, however, the interactions between the different components are not similar we can see positive or negative deviations from the
calculated vapor pressure. An actual vapor pressure greater than that predicted by Raoult's law is said to be a positive deviation
and an actual vapor pressure lower than that predicted by Raoult's law is a negative deviation.
Psoln = Prolv X Xolv
where Psoln is the vapor pressure of gaseous
solvent above the solution, Psoly is the vapor
pressure of the pure solvent, and Xsolv is the mole
fraction of the solvent.
Part A
Imagine a solution of two liquids in which the molecules interact less favorably than they do in the individual liquids. Will this
solution deviate positively from, deviate negatively from, or ideally follow Raoult's law?
An assumption of Raoult's law is that the
interactions between the different components of
the solution are similar in magnitude to the
interactions of each of the components with
themselves; in other words, the components form
an ideal solution. Deviations from this law are
observed when interactions between solute and
solvent are not similar.
• View Available Hint(s)
O t will deviate positively.
O It will deviate negatively.
O It will be an ideal solution.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT
