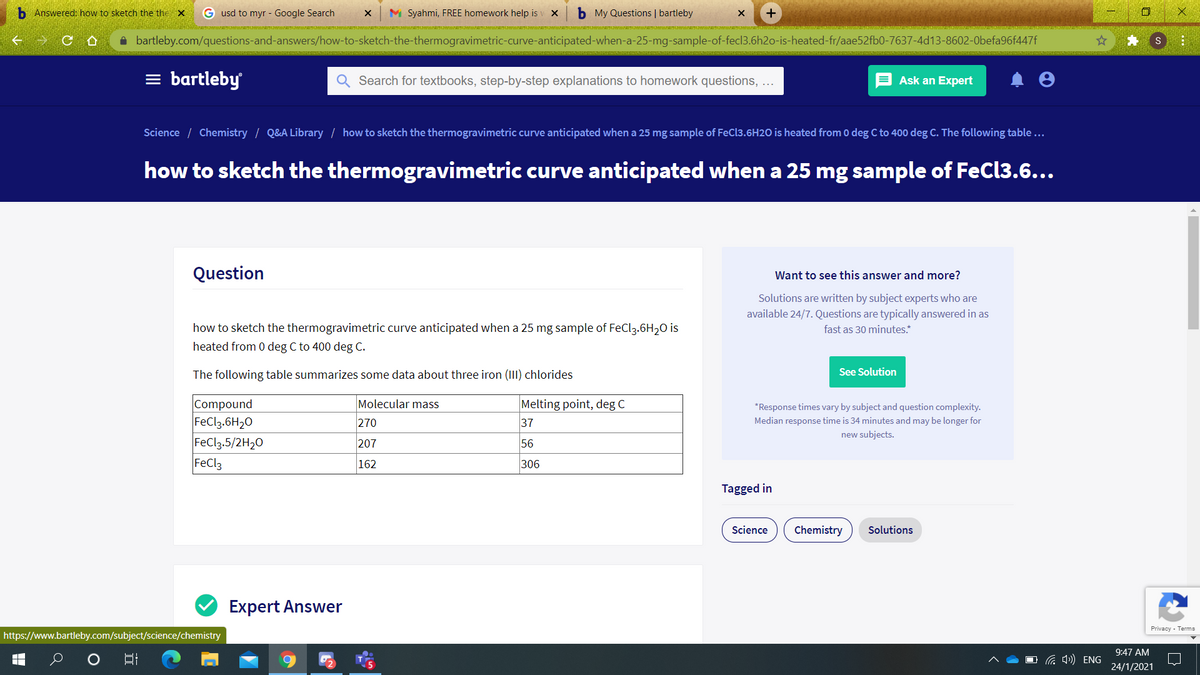
how to sketch the thermogravimetric curve anticipated when a 25 mg sample of FeCl,.6H20 is heated from 0 deg C to 400 deg C. The following table summarizes some data about three iron (III) chlorides Molecular mass Compound FeCl3.6H20 FeCl3.5/2H,0 FeCl3 Melting point, deg C 270 37 207 56 162 306
how to sketch the thermogravimetric curve anticipated when a 25 mg sample of FeCl,.6H20 is heated from 0 deg C to 400 deg C. The following table summarizes some data about three iron (III) chlorides Molecular mass Compound FeCl3.6H20 FeCl3.5/2H,0 FeCl3 Melting point, deg C 270 37 207 56 162 306
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter6: Properties Of Hydrates
Section: Chapter Questions
Problem 1ASA: A student is given a sample of a pink manganese (II) chloride hydrate. She weighs the sample in a...
Related questions
Question
100%
what is the answer for the question?
Transcribed Image Text:b Answered: how to sketch the the X
G usd to myr - Google Search
M Syahmi, FREE homework help is
b My Questions | bartleby
合
i bartleby.com/questions-and-answers/how-to-sketch-the-thermogravimetric-curve-anticipated-when-a-25-mg-sample-of-fecl3.6h2o-is-heated-fr/aae52fb0-7637-4d13-8602-0befa96f447f
= bartleby
Search for textbooks, step-by-step explanations to homework questions, ...
Ask an Expert
Science / Chemistry / Q&A Library / how to sketch the thermogravimetric curve anticipated when a 25 mg sample of FeCl3.6H2O is heated from 0 deg C to 400 deg C. The following table...
how to sketch the thermogravimetric curve anticipated when a 25 mg sample of FeCl3.6...
Question
Want to see this answer and more?
Solutions are written by subject experts who are
available 24/7. Questions are typically answered in as
how to sketch the thermogravimetric curve anticipated when a 25 mg sample of FeCl3.6H20 is
heated from 0 deg C to 400 deg C.
fast as 30 minutes.*
The following table summarizes some data about three iron (III) chlorides
See Solution
Compound
FeCl3.6H20
FeCl3.5/2H20
FeCl3
Molecular mass
Melting point, deg C
37
*Response times vary by subject and question complexity.
270
Median response time is 34 minutes and may be longer for
new subjects.
207
56
162
306
Tagged in
Science
Chemistry
Solutions
Expert Answer
Privacy - Terms
https://www.bartleby.com/subject/science/chemistry
9:47 AM
O G ) ENG
24/1/2021
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
