The molar heat capacity (Cpm (T)) for N2 is given by, P,m Cp.m (T)=(27.0+5.9×10T-0.34×10“T²)J mol' K Calculate AH when heating 1 mol of N2 from 25-125°C.
Q: (69,85) A 25g ice cube (pure water) at is placed into a 115g sample of pure water at 25 C. The…
A: Given: Mass of ice = 25 g. Initial temperature of ice = -10 oC Mass of water = 115 g. And initial…
Q: Example 3- 3 : In an air motor cylinder, the compressed air has an internal energy of 450 kJ/kg at…
A: Given that, the internal energy of the air motor cylinder at the beginning of the expansion = U1 =…
Q: Find ΔS for the system, surroundings, and universe when 28.2 g of liquid H2O is evapourated at 100.0…
A: Thermodynamics is a branch of chemistry that deals with the flow of heat energy. There are two…
Q: This pV diagram shows two different thermodynamic processes undergone by 0.40 mol of a monatomic gas…
A: In the given PV diagram, two different thermodynamic processes are undergone by: Number of moles =…
Q: To properly determine the internal energy of combustion methylhydrazine, the calorimeter was first…
A: Given the temperature change, ∆T = 1.98 K Mass of sucrose ignited = 0.4500 g Molar mass of sucrose…
Q: find the dH when 56g of AgNO3 (M = 169.9 g/mol) reacts. If the heat involved (derived from answer a)…
A: Given that ΔH of AgNO3 is -124.4 kJ/mol, at standard conditions, the ΔH is to be determined when 56g…
Q: 5.33 What is the difference between specific heat and heatcapacity? What are the units for these two…
A: The heat 'q' required to raise the temperature of a sample of mass 'm' having specific heat 'c' from…
Q: A 170.0g sample of metal at 83.00 degrees is added to 170.0g of H2O(l) at 15 degrees in an insultaed…
A: To solve this we need to use concept of heat loss and heat gained. In above problem, heat is lost by…
Q: 21.6 g of an acidic solution was mixed with 60.4 g of water in a calorimeter with a calorimeter…
A:
Q: 18. Estimate the standard heat of reaction (in kJ mol) for the following reaction CH4 (8) + 202(g) →…
A: For the following reaction CH4 (g) + 2O2 (g) —> CO2 (g) + 2H2O(g) The standard heats of…
Q: Find the Debye temperature for sodium chloride at 10, 15 and 20 K, where its corresponding specific…
A:
Q: The heat capacity (Cp) 0f an hal0genated 0rganic c0p0und, chl0r0f0rm is given by the f0ll0w…
A:
Q: B. Dissolution of Sodium Acetate Trihydrate a.547 1:250 Mass of water Mass of NaC2H3O2 3H20 Mass of…
A: The data given is,
Q: For Water at 15 bar and 400°C What is the specific internal energy in kJ/kg?
A:
Q: Calculate an approximate heat of combustion for ethane (C2H6) in kilojoules per mole by using the…
A: Combustion equation of ethane can be written as- 2C2H6 + 7O2 ----> 4CO2 + 6H2O
Q: ng becomes wet. She is packing emergency rations that if com- pletely metabolized will release 35 kJ…
A: Solution - Mass = 51 * 10^3g Specific heat = 4.2 Jg/k T = 2.5 K Formulas - Heat energy = q = Mass *…
Q: The Debye temperature of LiCl, OD, is 425K, calculate its molar heat capacity, Cy, at 5 K and at 500…
A: The Debye temperature of LiCl(QD) = 425 K
Q: A gas at pressure = 5 MPa is expanded from 123 in³ to 456 ft³. During the process heat = 789 kJ is…
A: Given: Pressure = 5 MPa = 5 x 106 Pa Initial volume = 123 inch3 Final volume = 456 ft3 1 inch3 =…
Q: 1. Real-life heat Capacity is temperature dependent. Consider the formation of water vapor H2(g)+(…
A: Given: Cp=a+bT+CT-2 ∆H298 K=-60 kCal
Q: A 25.0 g mass of ice [H2O (s)] at 273 K is added to 150.0 g of H20 (1) at 360 K at constant…
A: Molar mass of water = 18 g/mol Moles of ice = 25 / 18 = 1.4 moles Moles of water = 150 / 18 = 8.3…
Q: I am poor at physics chem, so please, explain it as much as possible! One mole of nitrogen (N2) is…
A: Isobaric process: Isobaric process is thermodynamic process in which the pressure stays constant. ΔP…
Q: Using the following equation and the standard enthalpies of formation given below, calculate the…
A:
Q: b) Recognizing then that the internal energy, U, is a state function of T and V, prove that for any…
A:
Q: 3. A 1.5 kg of liquid having a constant specific heat of 2.5 kj/kg.°C is stirred in a well…
A: Heat produced in insulated container is the internal energy.
Q: Between 0 °C and 100 °C, the heat capacity of Hg(l) is given by: Cp,m (Hg, l) [units: J mol-1 K-1]…
A: Specific heat (Cp,m) is the heat energy required to raise the temperature of a mole of a substance…
Q: A bomb calorimeter was calibrated by burning 0.610g benzoic acid. The temperature of calorimeter was…
A: Heat capacity of calorimeter can be calculated using formula: Q = m.C.∆T Where, Q = heat absorbed or…
Q: calculate the heat gained by the water: Q=CmΔT heat lost by sample: ms* cs *(Ts-T) = mw *cw (T…
A: Given data : known unknown (ms) mass of unknown= 240.40g (Cs) specific heat of sample: ? (Ts)…
Q: Calculate q, w, AE, and AH for the process in which 87.4 g of nitrous oxide gas (N₂O) is cooled from…
A:
Q: One mole of nitrogen (N2) is cooled from an initial temperature and pressure of 700 K and 10 bar to…
A: At constant pressure by Kirchhoff law
Q: How many kJ of energy need to be added to 50.00 g of Br2 (liq) to raise the temperature of the…
A: Given that : The mass of Br2 (liq) = 50.00 g Rise in temperature = 5.0 K The molar mass of Br2 =…
Q: A quantity of 4.275 g of an organic compound with a molecular formula C₁2H₂2 was burned in a…
A: we have to calculate the molar heat of combustion
Q: 3) The atomic heat capacity of solid Mo is given by the equation: cal 0.503x105 = 5.69 + 1.88x10-3T…
A:
Q: Show what percent T1 is of T2 for a heat engine whose ideal efficiency is 35%
A: Ideal efficiency ( η) of a heat engine is defined as the percentage of the heat energy which is…
Q: A gas in the initial state of p1 = 75 psia and V1 = 5 ft.° undergoes a process to p2 = 25 psia and…
A: Here we have provided change in enthalpy and we have to calculate the internal energy change. We…
Q: • Part A - How much heat (g) in Joules is absorbed when 1.00 g of liquid water is raised from 20.0°C…
A: Hii there, since there are multiple question posted. we are answering first-three questions. if you…
Q: The heat of combustion of liquid hexane (Ce H14) to carbon dioxide and liquid water at 298K is -4215…
A: Balanced equation is C6H14 (l) + 19/2 O2 (g) → 6 CO2 (g) + 7 H2O (l)…
Q: A piston/cylinder setup contains 1 kg of air at 20°C with an initial volume of V1=0.1 m3, as shown…
A:
Q: Calculate the standerd enthalpy chenge for the reaction 2CgHa- 1702g - 16COg- Hzon Given…
A: The enthalpy of a reaction calculated in a single step will be equal to the enthalpy of the reaction…
Q: where Tis in K and C, is in cal/(g mol)(K). 0. In a steady-state process, 10 g mol/s of O, at 100°C…
A:
Q: The heat (enthalpy) of combustion of methanol is ΔHc = - 726kJ at T = 298K, at 1atm. (R =…
A: The amount of heat energy is released when one mole of substance burns in presence of oxygen (air).…
Q: 3. Consider a 1.0 kg block of iron at 99°C placed in contact with a 1.0 kg block of iron at 25.0°C.…
A:
Q: A quantitity of 100mL of 0.500 MHCl was mixed with 50 mL of 0.500M NaOH in a constant pressure…
A: Given: Vol of HCl, = 100mL =0.100LConcentration of HCl = 0.500MVol of NaOH = 50mL…
Q: Aqueous ethylene glycol is commonly used in car radiators as an antifreeze and coolant. A 50%…
A: For Aqueous Ethylene Glycol, Specific heat capacity, Cs = 3.5 J/g ℃ mass, m = 4 kg = 4000 g Change…
Q: In rigid vessel, 19.4 moles of substance at 26.5 C is heated to 95.4C. Cv = 30.95 J/mol C Cp = 33.7…
A:
Q: ch of the following constants is/are needed to calculate the amor 70.5g of H20(s) at –35.0°C to…
A: The question is based on Hess law. it states that net enthalpy change for the reaction remains…
Q: Calculate an approximate heat of combustion for ethane (C, H6) in kilojoules per mole by using the…
A:
Q: The following information is given for diethyl ether at 1 atm: T-34.60°C AH (34.60°C) - 357.5 Jig…
A: Given data, melting point = -116.30 oC Boiling point = 34.6oC Specific heat of gas = 1.460 J/goC…
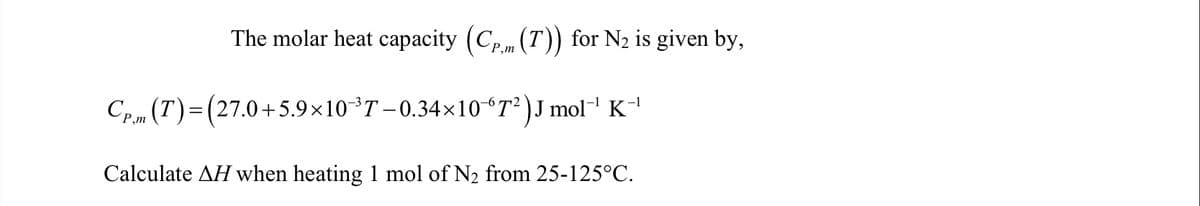
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

- To a test tube containing 10.0 ml of 0.50 M HCl solution in a calorimeter, 0.05482 g of solid MgO (MW = 40.30 g/mol) at the same temperature was added. A change in temperature of 6.2°C was recorded. In a separate experiment, the heat capacity of the calorimeter was determined to be 0.293 kJ/°C. 1. Calculate the experimental ΔHrxn (in kJ/mol) for the reaction between MgO andHCl.2. If the theoretical ΔHrxn of the reaction between MgO and HCl is -1349 kJ/mol, calculate the %error.A laboratory animal exercised on a treadmill, which, through pulleys, raised a mass of 200 g through 1.55 m. At the same time, the animal lost 5.0 J of energy as heat. Disregarding all other losses and regarding the animal as a closed system, what is its change in internal energy?A sample of a serum of mass 26.6 g is cooled from 290 K to 275 K at constant pressure by the extraction of 1.05 kJ of energy as heat. Calculate q and ΔH and estimate the heat capacity of the sample. report here the heat capacity = ________ J/k/g. 3 sig. number
- 2.8 g of a solid was dissolved in 51.4 g of a basic solution in a calorimeter with a calorimeter constant of 37.0 J K–1 with all substances initially at 81.5 °C.The resulting solution was observed to be at a temperature of 59.8 °C and have a heat capacity of 3.81 J g–1 K–1.Determine q for the dissolution process.Benzoic acid of mass 1.40 g is reacted with oxygen in a constant volume calorimeter to form H2O(l) and CO2(g) at 298 K. The mass of the water in the inner bath is 1.40×103g. The temperature of the calorimeter and its contents rises 2.84 K as a result of this reaction. Part A Calculate the calorimeter constant. The standard enthalpy of combustion of benzoic acid at 298 K is −3227 kJ⋅mol−1. CP,m(H2O,l)=75.3J⋅mol−1⋅K−1.a) A 1.60 dm3 sample of a mixture of methane gas, CH4 and oxygen gas, measured at 25 oC and 101 kPa, was allowed to react in a bomb calorimeter in which, had a heat capacity of 5.30 kJ/K altogether with its contents. The complete combustion of the methane gas to carbon dioxide gas and water caused a temperature rise in the calorimeter of 6.28 K. Given that ∆Ho c (CH4) is -560 kJ/mol. i. Define the standard enthalpy of combustion of methane ii. Write a thermochemical equation for the combustion of methane iii. Knowing the ∆Ho c of methane, calculate the number of mole of methane in the mixture. iv. Calculate the total moles of gases in the calorimeter.
- 1. Calculate the standard enthalpy of formation of C10H10. Its standard enthalpy of combustion is shown by the equation: 2C10H10 (l) + 25O2 (g) → 20CO2 (g) + 10H2O (l) ∆H = 10,314 KJ/mol The standard heat of formation (KJ/mol) at 298.15oC : CO2 = -393.5 ; H2O(l) = -285.8 2. Determine ΔG° at 298.15K for the reaction: 4Fe (s) + 3O2 (g) → 2Fe2O3 (s) Given that ΔH° = -1648 kJ ; ΔS° = -549.3 J/K 3. In the picture, Find the the ∆H°rxn if ∆G°rxn is 420 kJ/mol and ∆S°rxn is -143 J/mol*kAt low temperatures the heat capacity of Ag(s) is found to obey the Debye law Cp,m = aT3, with a = 1.956 × 10−4 J K−4 mol−1. Determine Sm(10 K) − Sm(0) for silver.a) What is the standard enthalpy of reaction per mole of either HCl or NaOH when 50.00 cm3 of 0.1 moldm-3 of hydrochloric acid (HCl) and 50.00 cm3 of 0.1 moldm-3 of sodium hydroxide (NaOH) are mixed? According to your simulation, the temperature rose by 0.68 oC. (Density of water = 1gcm-3, Specific heat capacity of water = 4.18 J g-1 oC-1) Show your working below:
- At constant pressure and 25C, what is enthalpy for the reaction: 2C2H6 + 7O2 -> 4CO2 + H2O, if the complete consumption of 12g of C2H6 liberates - 700kJ of heat energy? (-3508 kJ)The complete combustion of solid fumaric acid, HOOCCH=CHCOOH, in a bomb calorimeter released 1333 kJ mol -1 of heat at 298 K. Calculate (a) the internal energy of combustion, (b) the enthalpy of combustion . (c) Usetabulated values for the standard enthalpy of formation of water and carbon dioxide to calculate the enthalpy of formation of solid fumaric acid.To properly determine the internal energy of combustion methylhydrazine, the calorimeter was first calibrated. A 0.4500 g sample of sucrose (MW 342.296 g/mol)) was ignited under identical conditions and produced a temperature rise of 1.98 K. For sucrose, the internal energy combustion at constant volume, ∆U, is known to be –5616.64 kJ/mol. Calculate the calorimeter constant, in kJ/K.

