How would the d²-y² orbital in the n = 5 shell compare to the dx²-y² orbital in the n = 3 subshell? A. The contour of the orbital would extend further out along the x and y axes. B. The value of l would increase by 2. C. The radial probability function would include two more nodes. D. The orientation of the orbital would be rotated 45° along the xy plane. E. The me value would be the same. Drag the appropriate items to their respective bins. ► View Available Hint(s) Reset A B C D E True False Help
How would the d²-y² orbital in the n = 5 shell compare to the dx²-y² orbital in the n = 3 subshell? A. The contour of the orbital would extend further out along the x and y axes. B. The value of l would increase by 2. C. The radial probability function would include two more nodes. D. The orientation of the orbital would be rotated 45° along the xy plane. E. The me value would be the same. Drag the appropriate items to their respective bins. ► View Available Hint(s) Reset A B C D E True False Help
Chapter7: Atomic Structure And Periodicity
Section: Chapter Questions
Problem 144AE: Although no currently known elements contain electrons in g orbitals in the ground state, it is...
Related questions
Question
Pls help ASAP. Pls do both of the asked parts.
Transcribed Image Text:-
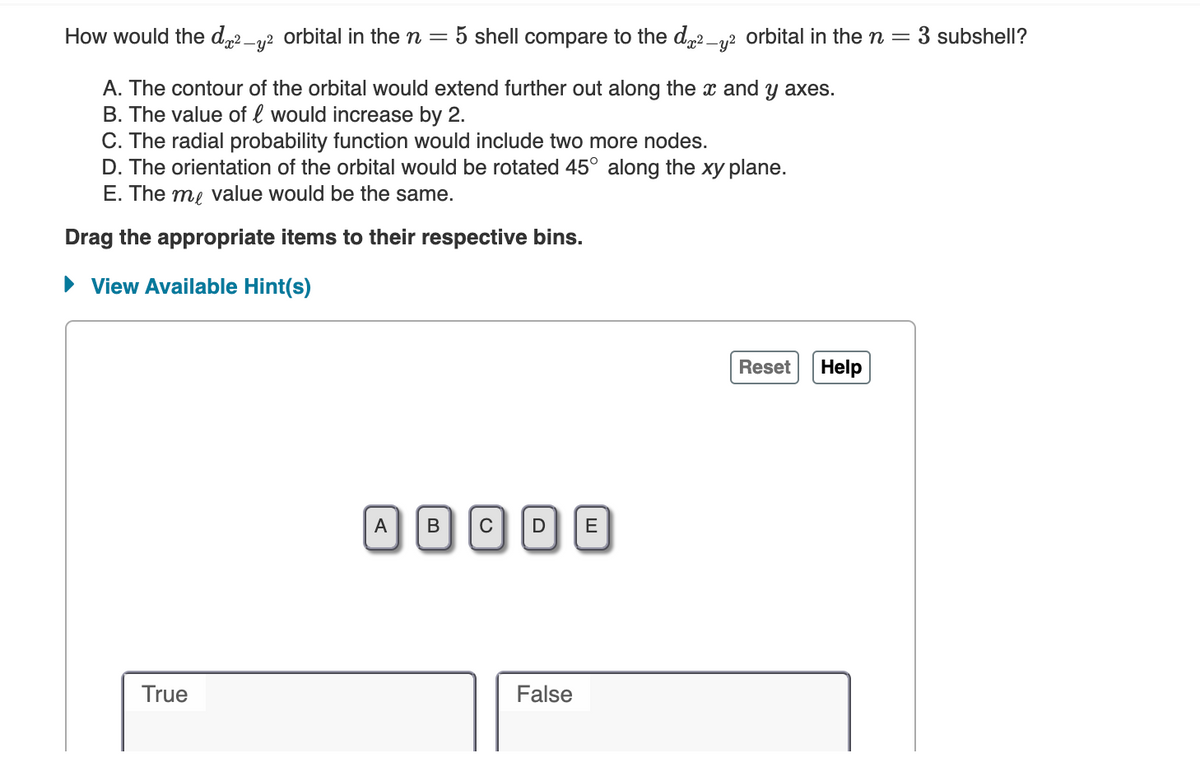
How would the dx²-y² orbital in the n= 5 shell compare to the d²-² orbital in the n 3 subshell?
A. The contour of the orbital would extend further out along the x and y axes.
B. The value of l would increase by 2.
C. The radial probability function would include two more nodes.
D. The orientation of the orbital would be rotated 45° along the xy plane.
E. The me value would be the same.
Drag the appropriate items to their respective bins.
► View Available Hint(s)
Reset
A BCD E
True
False
Help
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning