- HS (ag) +H*(aq), 1s2- (ag) + H*(ag), K2 = 1.21x10-19 brium constant Krinal for the following reaction? K = 9 27x10-8, and s2-(49) + 21 wer numerically. le Hint(s) ?
- HS (ag) +H*(aq), 1s2- (ag) + H*(ag), K2 = 1.21x10-19 brium constant Krinal for the following reaction? K = 9 27x10-8, and s2-(49) + 21 wer numerically. le Hint(s) ?
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter16: Solubility And Precipitation Equilibria
Section: Chapter Questions
Problem 50P
Related questions
Question
4.
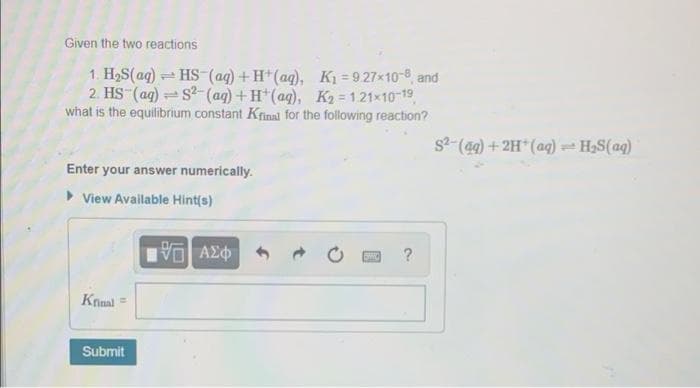
Transcribed Image Text:Given the two reactions
1. H2S(ag) HS-(ag) +H*(ag), K = 9.27x10-8, and
2. HS (ag) s-(aq)+H*(aq), K2 = 121x10-19,
what is the equilibrium constant Krinal for the following reaction?
s2-(49) + 2H*(aq) - H,S(aq)
Enter your answer numerically.
View Available Hint(s)
?
Krinal =
Submit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning