https:/ ession.masteringchemistry.com/myct/itemviewrassighn 144497960&attemptNo= I worites here, select then *, and drag to the Favorites Bar folder. Or import from another browser. Import favorites a; K.M.T., Pressure and Partial Pressure (text sections 8.3, 8.4 and 8.11) Spr 20 - Attempt 1 m 8.20 13 of 13 Part A Determine the percent composition of air in the lungs from the following composition in partial pressures: PN, = 577mmHg, Po, = 96mmHg, Pco, = 40mmHg, PH,0 = 47mmHg; all at 37°C and 1 atm pressure. Πν ΑΣφ 72.8 % N2 Submit Previous Answers Request Answer X Incorrect; Try Again; 4 attempts remaining Part B Nν ΑΣφ % O2 Submit Reauest Answer Problem 8.20 Part C να ΑΣφ % CO2 Submit Request Answer Part D Πν ΑΣφ % H2O Submit Request Answer
https:/ ession.masteringchemistry.com/myct/itemviewrassighn 144497960&attemptNo= I worites here, select then *, and drag to the Favorites Bar folder. Or import from another browser. Import favorites a; K.M.T., Pressure and Partial Pressure (text sections 8.3, 8.4 and 8.11) Spr 20 - Attempt 1 m 8.20 13 of 13 Part A Determine the percent composition of air in the lungs from the following composition in partial pressures: PN, = 577mmHg, Po, = 96mmHg, Pco, = 40mmHg, PH,0 = 47mmHg; all at 37°C and 1 atm pressure. Πν ΑΣφ 72.8 % N2 Submit Previous Answers Request Answer X Incorrect; Try Again; 4 attempts remaining Part B Nν ΑΣφ % O2 Submit Reauest Answer Problem 8.20 Part C να ΑΣφ % CO2 Submit Request Answer Part D Πν ΑΣφ % H2O Submit Request Answer
Chapter3: Using Spreadsheets In Analytical Chemistry
Section: Chapter Questions
Problem 3.4QAP
Related questions
Question
100%
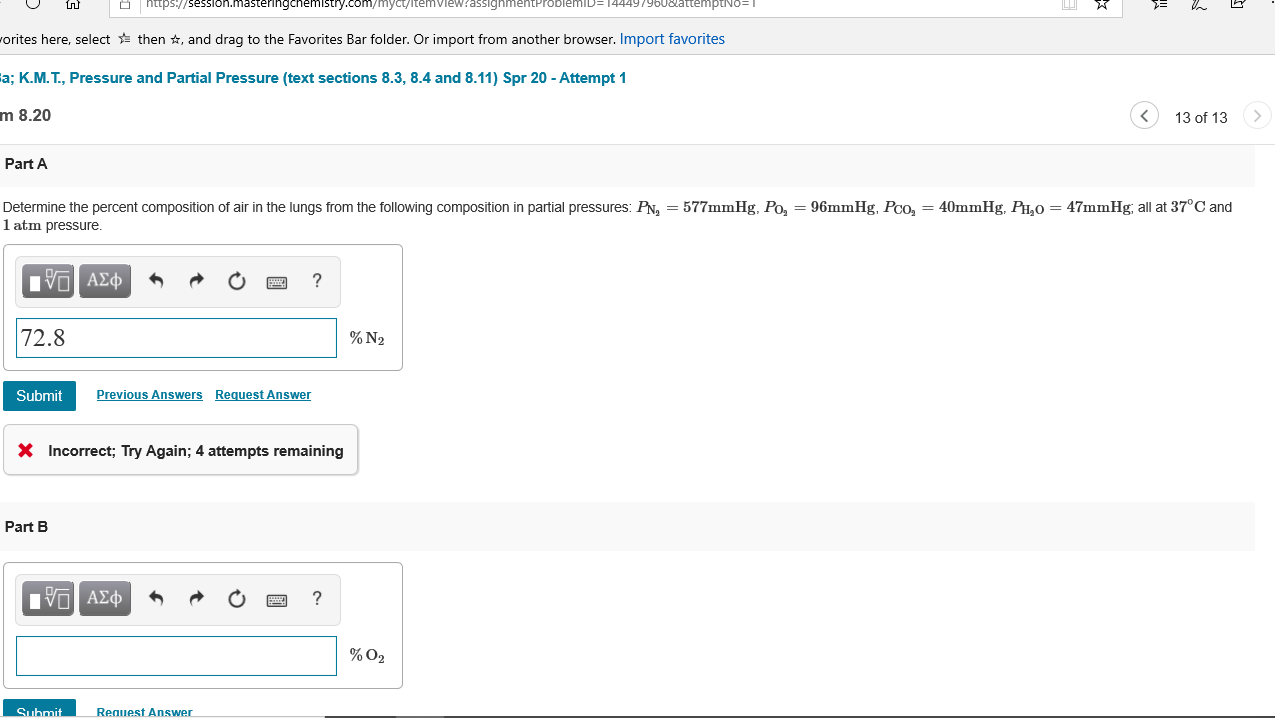
Determine the percent composition of air in the lungs from the following composition in partial pressures: PN2=577mmHg, PO2=96mmHg, PCO2=40mmHg, PH2O=47mmHg; all at 37∘C and 1atm pressure.
Transcribed Image Text:https:/
ession.masteringchemistry.com/myct/itemviewrassighn
144497960&attemptNo= I
worites here, select then *, and drag to the Favorites Bar folder. Or import from another browser. Import favorites
a; K.M.T., Pressure and Partial Pressure (text sections 8.3, 8.4 and 8.11) Spr 20 - Attempt 1
m 8.20
13 of 13
Part A
Determine the percent composition of air in the lungs from the following composition in partial pressures: PN, = 577mmHg, Po, = 96mmHg, Pco, = 40mmHg, PH,0 = 47mmHg; all at 37°C and
1 atm pressure.
Πν ΑΣφ
72.8
% N2
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 4 attempts remaining
Part B
Nν ΑΣφ
% O2
Submit
Reauest Answer
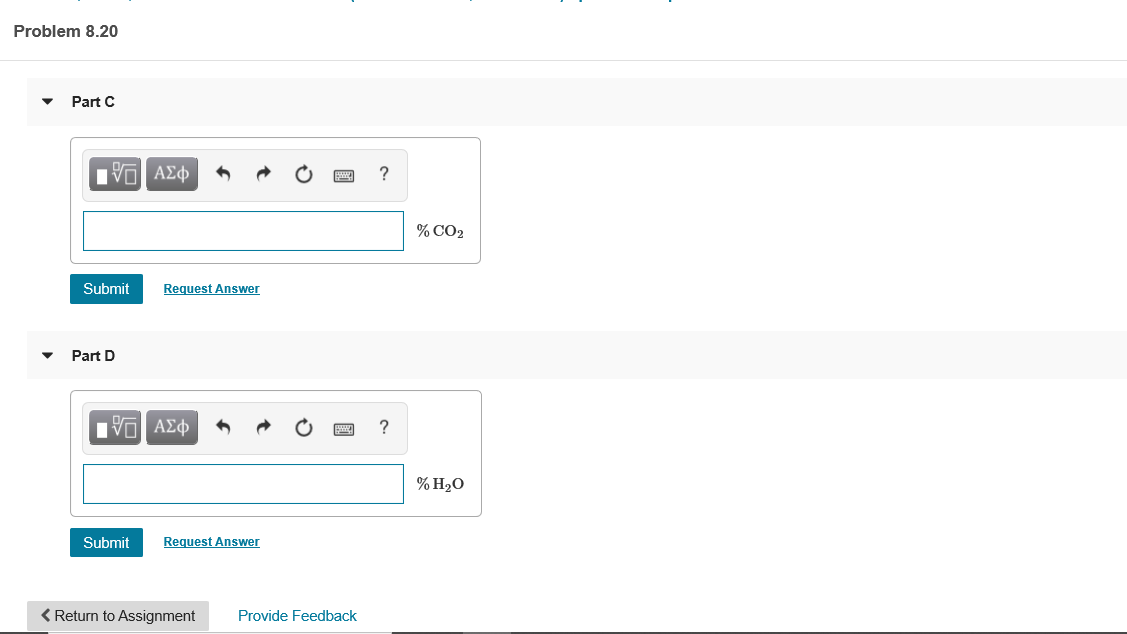
Transcribed Image Text:Problem 8.20
Part C
να ΑΣφ
% CO2
Submit
Request Answer
Part D
Πν ΑΣφ
% H2O
Submit
Request Answer
<Return to Assignment
Provide Feedback
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

