Hydrogen 8+ 8n Oxygen Hydrogen The illustrations above show molecular structures of multiple water molecules (left side box) and a single water molecule (right side bax). Use these graphics to answer the following question. bonds are present between the Hydrogens and Oxygen. However, between water molecules, weak bonds form contributie In a water molecule, to the properties of cohesion and adhesion. lonic; hydrogen covalent; hydrogen hydrogen; covalent covalent: lonic 0000
Hydrogen 8+ 8n Oxygen Hydrogen The illustrations above show molecular structures of multiple water molecules (left side box) and a single water molecule (right side bax). Use these graphics to answer the following question. bonds are present between the Hydrogens and Oxygen. However, between water molecules, weak bonds form contributie In a water molecule, to the properties of cohesion and adhesion. lonic; hydrogen covalent; hydrogen hydrogen; covalent covalent: lonic 0000
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter13: Gases
Section: Chapter Questions
Problem 66QAP
Related questions
Question
Could you please assist?
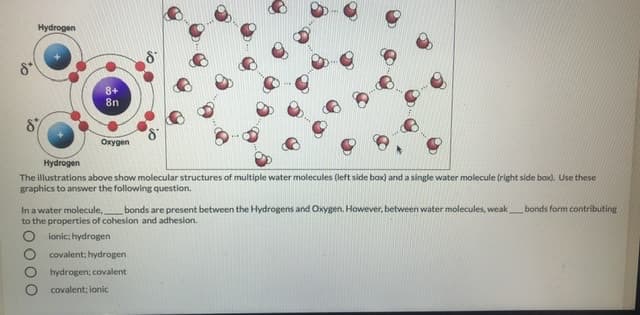
Transcribed Image Text:Hydrogen
8+
8n
Oxygen
Hydrogen
The illustrations above show molecular structures of multiple water molecules (left side box) and a single water molecule (right side bax). Use these
graphics to answer the following question.
bonds are present between the Hydrogens and Oxygen. However, between water molecules, weak bonds form contributie
In a water molecule,
to the properties of cohesion and adhesion.
lonic; hydrogen
covalent; hydrogen
hydrogen; covalent
covalent: lonic
0000
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning