i ezto.mheducation.com/ext/ Saved Module 1 Exam Packaged cake mixes usually contain baking powder, a mixture of sodium hydrogen carbonate and calcium hydrogen phosphate that react to produce carbon dioxide gas when they are heated in water. The CO2(g) formed allows the cake to "rise." When such cake mixes are used at high altitudes, often the cake will rise too much and collapse, unless special instructions are followed. Why does this happen? 12 Multiple Choice Due to the higher atmospheric pressure, a greater volume of carbon dioxide is created. Due to the reduced atmospheric pressure, less volume of carbon dioxide is created. Due to the reduced atmospheric pressure, a greater volume of carbon dioxide is created. Mc Graw Hill < Prev 12 of 23 O Type bere to search Next >
i ezto.mheducation.com/ext/ Saved Module 1 Exam Packaged cake mixes usually contain baking powder, a mixture of sodium hydrogen carbonate and calcium hydrogen phosphate that react to produce carbon dioxide gas when they are heated in water. The CO2(g) formed allows the cake to "rise." When such cake mixes are used at high altitudes, often the cake will rise too much and collapse, unless special instructions are followed. Why does this happen? 12 Multiple Choice Due to the higher atmospheric pressure, a greater volume of carbon dioxide is created. Due to the reduced atmospheric pressure, less volume of carbon dioxide is created. Due to the reduced atmospheric pressure, a greater volume of carbon dioxide is created. Mc Graw Hill < Prev 12 of 23 O Type bere to search Next >
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter17: Equilibrium
Section: Chapter Questions
Problem 33QAP: . For the reaction system C(s)+H2O(g)H2(g)+CO(g)which has already reached a state of equilibrium,...
Related questions
Question
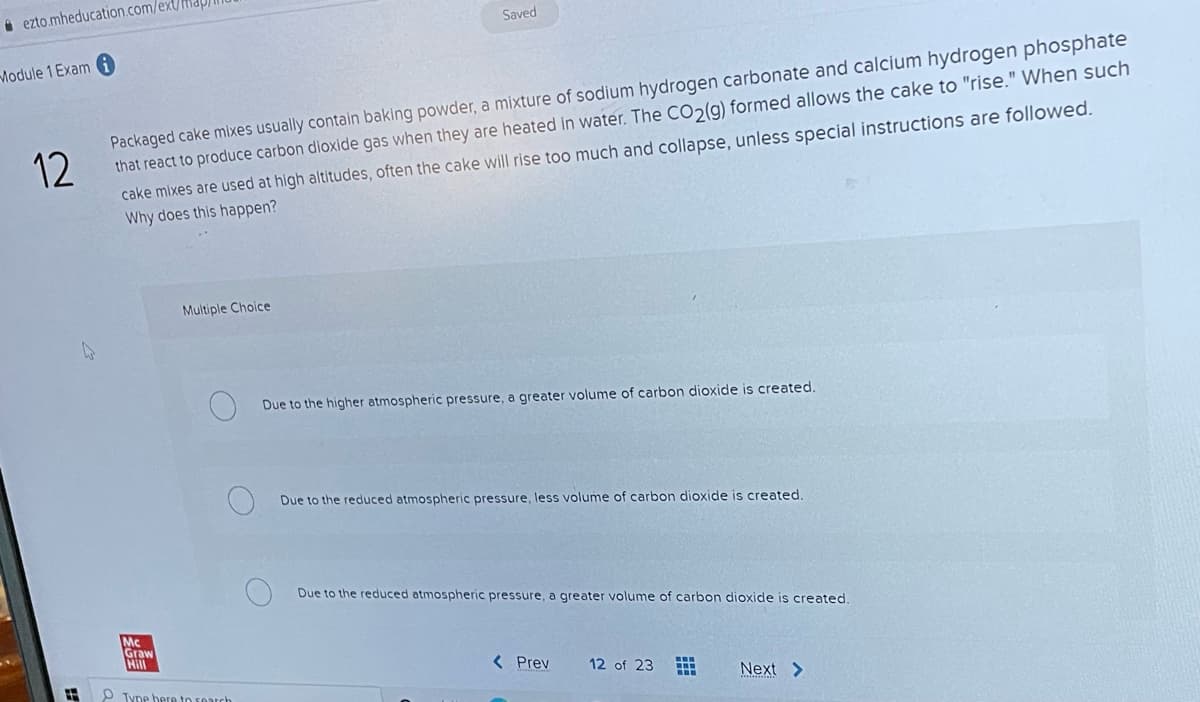
Transcribed Image Text:i ezto.mheducation.com/ext/
Saved
Module 1 Exam
Packaged cake mixes usually contain baking powder, a mixture of sodium hydrogen carbonate and calcium hydrogen phosphate
that react to produce carbon dioxide gas when they are heated in water. The CO2(g) formed allows the cake to "rise." When such
cake mixes are used at high altitudes, often the cake will rise too much and collapse, unless special instructions are followed.
Why does this happen?
12
Multiple Choice
Due to the higher atmospheric pressure, a greater volume of carbon dioxide is created.
Due to the reduced atmospheric pressure, less volume of carbon dioxide is created
Due to the reduced atmospheric pressure, a greater volume of carbon dioxide is created.
Mc
Graw
Hill
< Prev
12 of 23
O Type bere to search
Next >
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
