Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter14: Mixtures And Solutions
Section14.3: Factors Affecting Solvation
Problem 42SSC
Related questions
Question
100%
I have to figure out van't Hoff factor from the following information. I tried 1.0 and 2.0 but it says I am wrong.
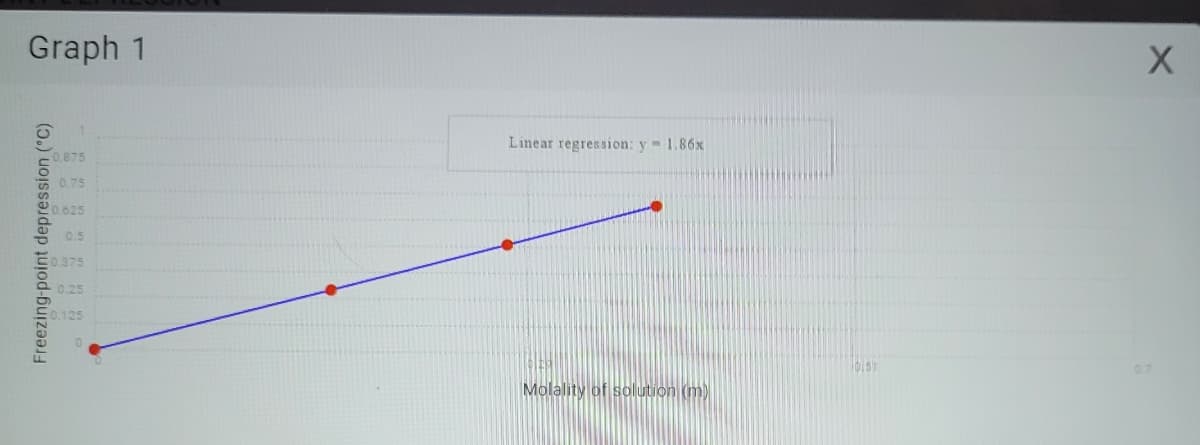
Transcribed Image Text:Graph 1
Freezing-point depression (°C)
0.875
0.75
0.625
0.5
0.375
0.25
125
Linear regression: y = 1.86x
Molality of solution (m)
bist
X
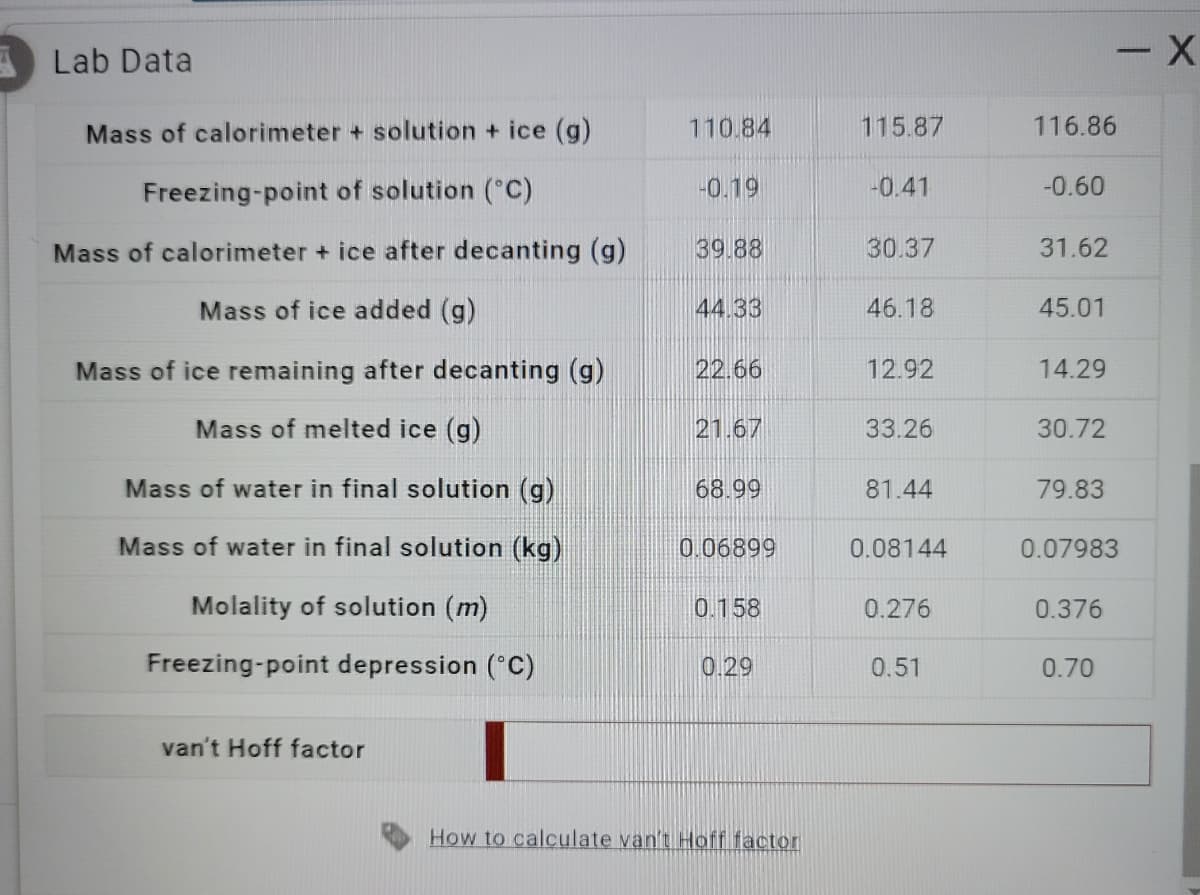
Transcribed Image Text:Lab Data
Mass of calorimeter + solution + ice (g)
Freezing-point of solution (°C)
Mass of calorimeter + ice after decanting (g)
Mass of ice added (g)
Mass of ice remaining after decanting (g)
Mass of melted ice (g)
Mass of water in final solution (g)
Mass of water in final solution (kg)
Molality of solution (m)
Freezing-point depression (°C)
van't Hoff factor
110.84
-0.19
39.88
44.33
22.66
21.67
68.99
0.06899
0.158
0.29
How to calculate van't Hoff factor
115.87
-0.41
30.37
46.18
12.92
33.26
81.44
0.08144
0.276
0.51
116.86
-0.60
31.62
45.01
14.29
30.72
79.83
- X
0.07983
0.376
0.70
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,
