I need solutions Q a, c,d 5.a. Write the equation for the dissolution of a Metal(II) chloride in water as well as the expression for Ksp. b. Calculate the solubility(s) and the solubility product, Ksp, for a Metal(II) chloride, if 50.0 mL of a saturated solution of Metal(II) chloride was found to contain 0.2207 g of Metal(II) chloride dissolved in it (Molar mass of M(II)Cl2 = 278.1g/mol, T = 250C). c. Based on the given molar mass, calculate the atomic mass of the metal. d. Identify the metal. Write the formula of its chloride salt.
I need solutions Q a, c,d
5.a. Write the equation for the dissolution of a Metal(II) chloride in water as well as the
expression for Ksp.
b. Calculate the solubility(s) and the solubility product, Ksp, for a Metal(II) chloride, if 50.0 mL of
a saturated solution of Metal(II) chloride was found to contain 0.2207 g of Metal(II) chloride
dissolved in it (Molar mass of M(II)Cl2 = 278.1g/mol, T = 250C).
c. Based on the given molar mass, calculate the
d. Identify the metal. Write the formula of its chloride salt.
e. Discuss the effect of addition of few drops of HCl to the previous system in equilibrium at the
same temperature.
The solubility product constant (Ksp) represents the solubility of products at equilibrium for solids in an aqueous solution.
a.
The chemical equation for dissolution of metal (II) chloride in water is represented as follows:
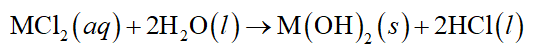
As the ionization of Metal (II) chloride will give metal hydroxide as solid. Thus, the solubility constant will be represented as follows:
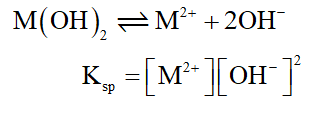
Step by step
Solved in 4 steps with 3 images









