Calculate the solublity at 25 °C of AgCl in pure water and In a 0.0040M AGNO, solution. You'll find K data In the ALEKS Data tab. sp Round both of your answers to 2 significant digits. solubility in pure water: solubility in 0.0040 M AGN0, solution:
Calculate the solublity at 25 °C of AgCl in pure water and In a 0.0040M AGNO, solution. You'll find K data In the ALEKS Data tab. sp Round both of your answers to 2 significant digits. solubility in pure water: solubility in 0.0040 M AGN0, solution:
Chapter6: The Systematic Approach To Equilibria: Solving Many Equations
Section: Chapter Questions
Problem 10P
Related questions
Question
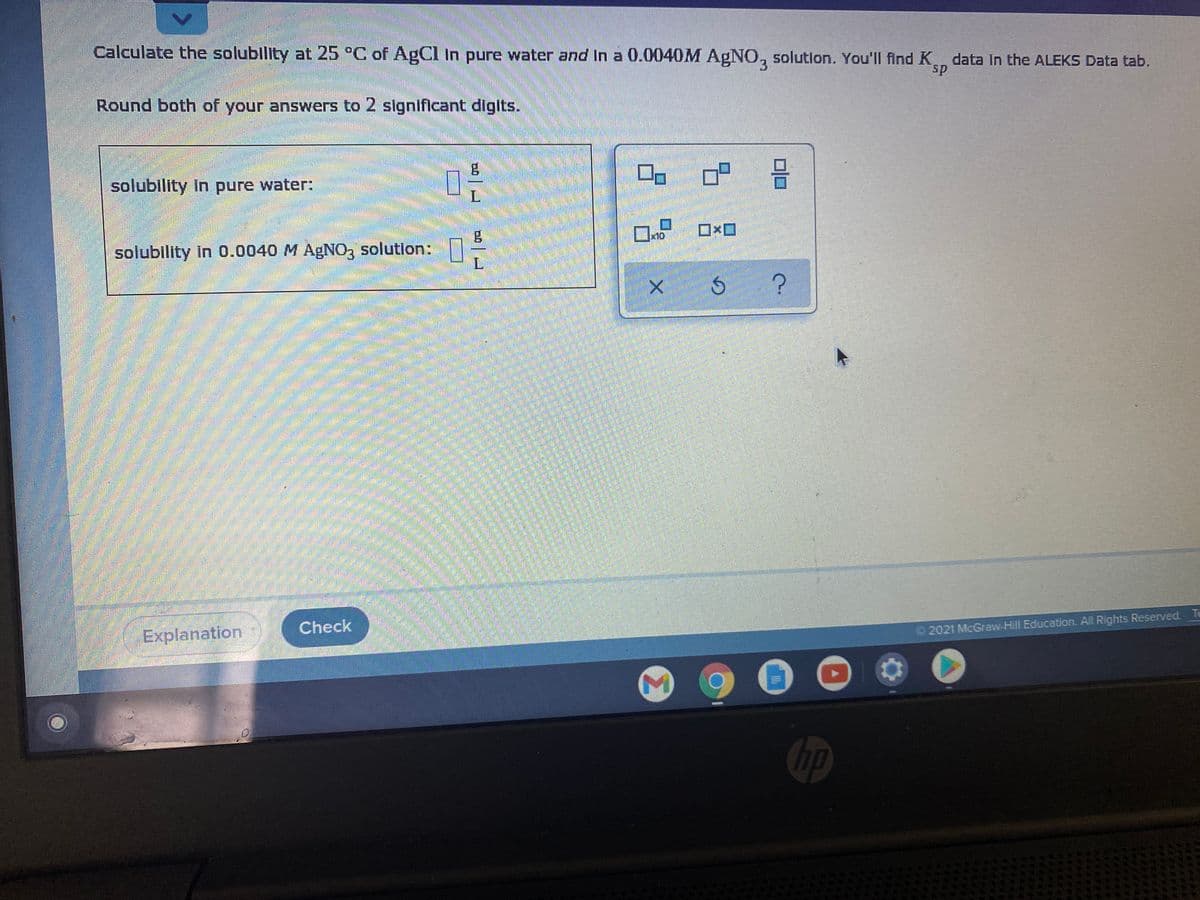
Transcribed Image Text:Calculate the solubility at 25 °C of AgCl In pure water and In a 0.0040M AgN0, solution. You'll find K data In the ALEKS Data tab.
3
sp
Round both of your answers to 2 significant digits.
solubility in pure water:
x10
solubility In 0.0040 M AgNO, solution:
L.
Check
©2021 McGraw-Hill Education. All Rights Reserved Te
Explanation
Chp
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

