i) Provide curly arrows describing both the forward and backward reactions for the ionisation of propanoic acid in water (see below). CO,H H20 H30 ii) The pKa value for this equilibrium process at 25 °C is 4.8. Using an equation show how this value relates to K, and use this information to comment on the acidity of carboxylic acids compared to hydrochloric acid (pK, = -7). ii) Predict whether substituted propanoic acids A and B are more, or less acidic than propanoic acid. Explain briefly your reasoning. A в CO,H CO2H
i) Provide curly arrows describing both the forward and backward reactions for the ionisation of propanoic acid in water (see below). CO,H H20 H30 ii) The pKa value for this equilibrium process at 25 °C is 4.8. Using an equation show how this value relates to K, and use this information to comment on the acidity of carboxylic acids compared to hydrochloric acid (pK, = -7). ii) Predict whether substituted propanoic acids A and B are more, or less acidic than propanoic acid. Explain briefly your reasoning. A в CO,H CO2H
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter15: Principles Of Chemical Reactivity: Equilibria
Section15.6: Disturbing A Chemical Equilibrium
Problem 2.2ACP
Related questions
Question
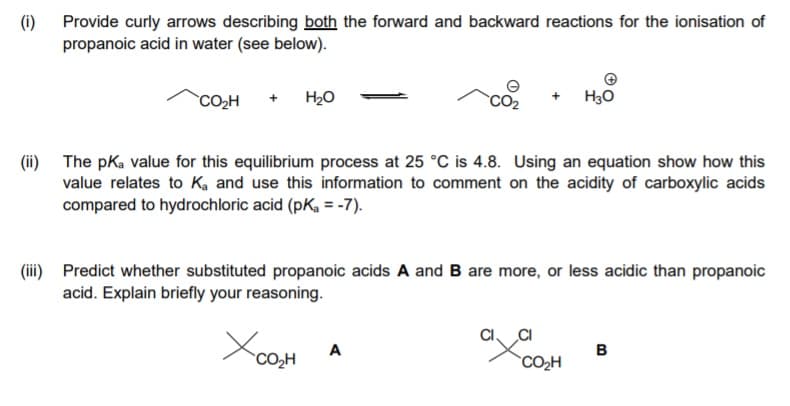
Transcribed Image Text:(i)
Provide curly arrows describing both the forward and backward reactions for the ionisation of
propanoic acid in water (see below).
`CO2H
+ H2O
H30
(ii) The pka value for this equilibrium process at 25 °C is 4.8. Using an equation show how this
value relates to Ka and use this information to comment on the acidity of carboxylic acids
compared to hydrochloric acid (pK, = -7).
(ii) Predict whether substituted propanoic acids A and B are more, or less acidic than propanoic
acid. Explain briefly your reasoning.
CI
A
B
CO,H
`CO2H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning