PLEASE GO THROUGH AND HELP ME. -the concentration of standard sodium thiosulphate solution is 0.01152 M. -the temperature of the equilibrium mixture in degree Celsius is 20oC.
PLEASE GO THROUGH AND HELP ME. -the concentration of standard sodium thiosulphate solution is 0.01152 M. -the temperature of the equilibrium mixture in degree Celsius is 20oC.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter14: Acid- Base Equilibria
Section: Chapter Questions
Problem 118CP: Malonic acid (HO2CCH2CO2H) is a diprotic acid. In the titration of malonic acid w ith NaOH,...
Related questions
Question
PLEASE GO THROUGH AND HELP ME.
-the concentration of standard sodium thiosulphate solution is 0.01152 M.
-the temperature of the equilibrium mixture in degree Celsius is 20oC.
In Determination #1:
Volume of thiosulphate = 32.50 mL
Volume of Aqueous aliquot = 10.15 mL
Strength of Thiosulphate = 0.01152 M
In Determination #2:
Volume of thiosulphate = 32.50 mL
Volume of Aqueous aliquot = 10.23 mL
Strength of Thiosulphate = 0.01152 M
In Determination #3:
Volume of thiosulphate = 32.67 mL
Volume of Aqueous aliquot = 10.31 mL
Strength of Thiosulphate = 0.01152 M
https://drive.google.com/file/d/1824fGKn1AMdByzDsSLq8Nv323rpC4e79/view?usp=sharing
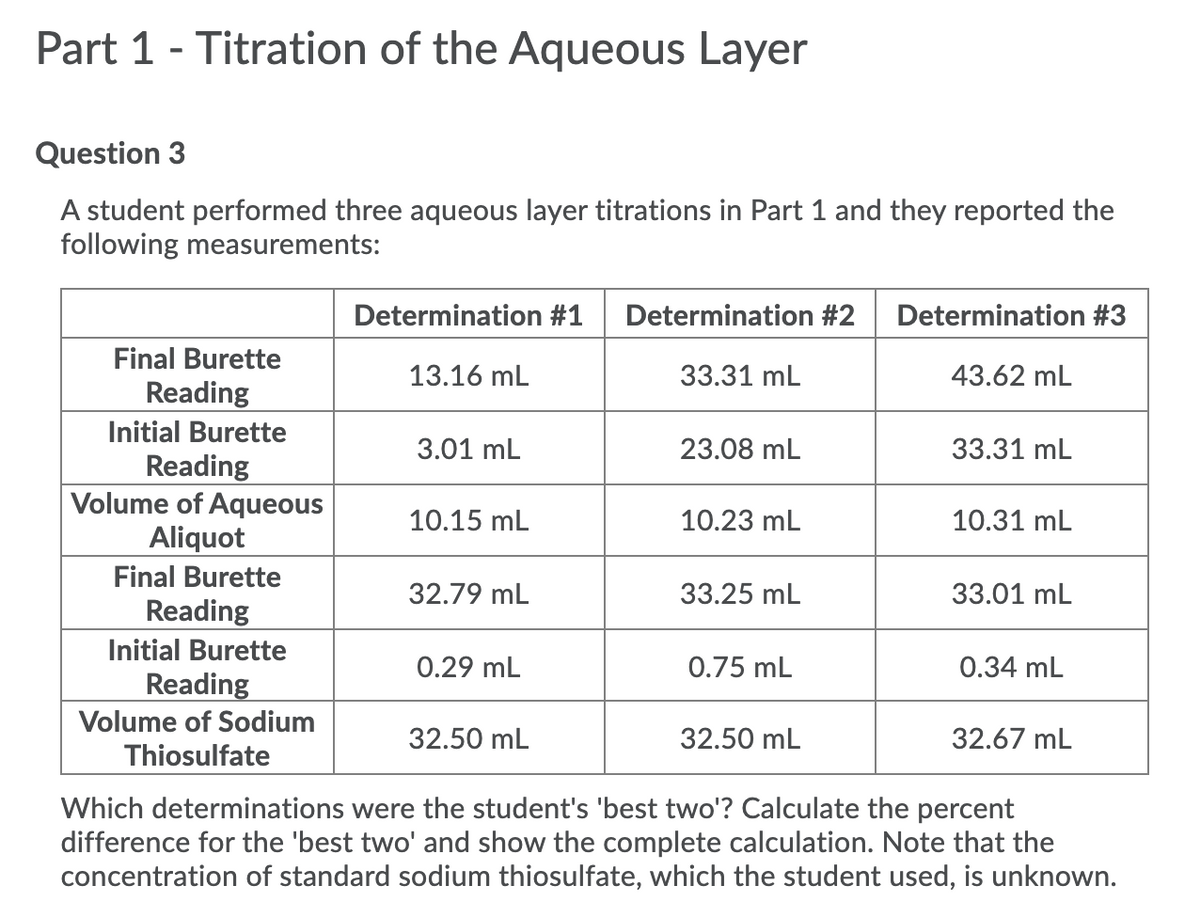
Transcribed Image Text:Part 1 - Titration of the Aqueous Layer
Question 3
A student performed three aqueous layer titrations in Part 1 and they reported the
following measurements:
Determination #1
Determination #2
Determination #3
Final Burette
13.16 mL
33.31 mL
43.62 mL
Reading
Initial Burette
3.01 mL
23.08 mL
33.31 mL
Reading
Volume of Aqueous
Aliquot
10.15 mL
10.23 mL
10.31 mL
Final Burette
32.79 mL
33.25 mL
33.01 mL
Reading
Initial Burette
0.29 mL
0.75 mL
0.34 mL
Reading
Volume of Sodium
32.50 mL
32.50 mL
32.67 mL
Thiosulfate
Which determinations were the student's 'best two'? Calculate the percent
difference for the 'best two' and show the complete calculation. Note that the
concentration of standard sodium thiosulfate, which the student used, is unknown.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning