I Rev Snapshots of two hypothetical reactions, A(g) + B(g) = AB(g) and X(g) + Y(g) at five different times are shown here. = XY(g) Part A t = 10 s 20 s 30 s 40 s 50 s Which reaction has a larger equilibrium constant? A(g) + B(g) = AB(9) O X(9) + Y(g) = XY(g) A(g) + B(g)= AB(g) Submit Request Answer t = 10 s 20 s 30 s 40 s 50 s Provide Feedback X(g) + Y(g)= XY(g)
I Rev Snapshots of two hypothetical reactions, A(g) + B(g) = AB(g) and X(g) + Y(g) at five different times are shown here. = XY(g) Part A t = 10 s 20 s 30 s 40 s 50 s Which reaction has a larger equilibrium constant? A(g) + B(g) = AB(9) O X(9) + Y(g) = XY(g) A(g) + B(g)= AB(g) Submit Request Answer t = 10 s 20 s 30 s 40 s 50 s Provide Feedback X(g) + Y(g)= XY(g)
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter13: Fundamental Equilibrium Concepts
Section: Chapter Questions
Problem 12E: Show that the complete chemical equation, the total ionic equation, and the net ionic equation for...
Related questions
Question
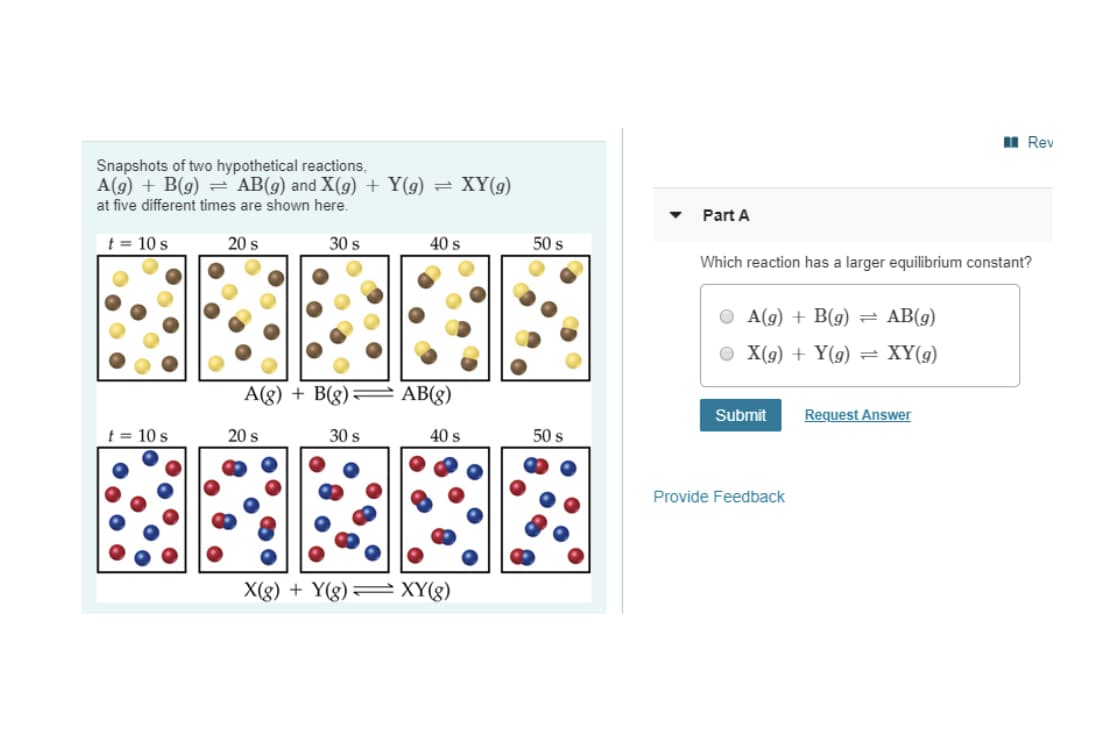
Transcribed Image Text:I Rev
Snapshots of two hypothetical reactions,
A(g) + B(g) = AB(g) and X(g) + Y(g)
= XY(g)
at five different times are shown here.
Part A
t = 10 s
20 s
30 s
40 s
50 s
Which reaction has a larger equilibrium constant?
О A() + B(9)
= AB(g)
O X(9) + Y(g)
XY(9)
A(g) + B(g)= AB(g)
Submit
Request Answer
t = 10 s
20 s
30 s
40 s
50 s
Provide Feedback
X(g) + Y(g)= XY(g)
Expert Solution
Step 1
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning