I Review | Constants Cyclopropane rearranges to form propene in the gas phase. Part A If the initial cyclopropane concetration is 0.0415 M, what is the cyclopropane concentration after 280 minutes? Express your answer with the appropriate units. CH2 CH3–CH=CH, Value Units H;C-CH; The reaction is first order in cyclopropane and has a measured rate constant of 3.36 x 10- s1 at 720 K. Submit Request Answer You may want to reference (Pages 598 - 605) Section 14.5 while completing this problem.
I Review | Constants Cyclopropane rearranges to form propene in the gas phase. Part A If the initial cyclopropane concetration is 0.0415 M, what is the cyclopropane concentration after 280 minutes? Express your answer with the appropriate units. CH2 CH3–CH=CH, Value Units H;C-CH; The reaction is first order in cyclopropane and has a measured rate constant of 3.36 x 10- s1 at 720 K. Submit Request Answer You may want to reference (Pages 598 - 605) Section 14.5 while completing this problem.
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter20: Kinetics
Section: Chapter Questions
Problem 20.38E: List at least four experimentally determined parameters that you, an experimenter, can define when...
Related questions
Question
100%
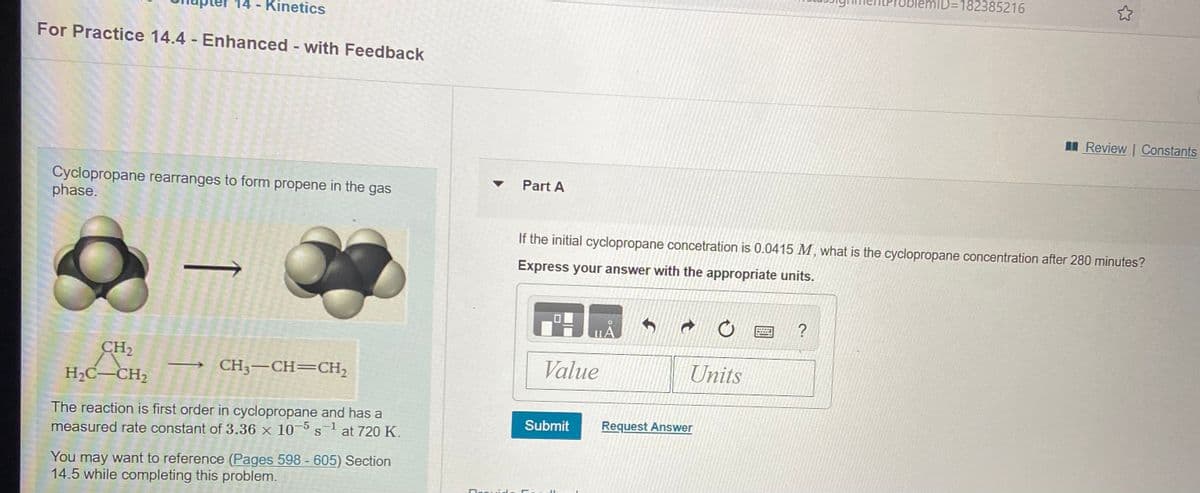
Transcribed Image Text:ID=182385216
Kinetics
For Practice 14.4 - Enhanced - with Feedback
I Review | Constants
Cyclopropane rearranges to form propene in the gas
phase.
v Part A
If the initial cyclopropane concetration is 0.0415 M, what is the cyclopropane concentration after 280 minutes?
Express your answer with the appropriate units.
CH2
CH3-CH=CH,
Value
Units
H2C-CH2
The reaction is first order in cyclopropane and has a
at 720 K.
Submit
Request Answer
measured rate constant of 3.36 x 10-5
You may want to reference (Pages 598 - 605) Section
14.5 while completing this problem.
Dreuid.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,
