I. Fill in the blanks. Hydrogen (H) has only 1 valence electron. So the noble gas closest to it is ____ One (1) hydrogen atom needs ___ electron to attain the same number of electrons with Helium. H2 represents ___ atoms of hydrogen. H H shows __ electrons being shared. (how many electrons being shared). Fluorine (F) has 7 valence electrons. How many valence electrons it should have to attain stability?
Atomic Structure
The basic structure of an atom is defined as the component-level of atomic structure of an atom. Precisely speaking an atom consists of three major subatomic particles which are protons, neutrons, and electrons. Many theories have been stated for explaining the structure of an atom.
Shape of the D Orbital
Shapes of orbitals are an approximate representation of boundaries in space for finding electrons occupied in that respective orbital. D orbitals are known to have a clover leaf shape or dumbbell inside where electrons can be found.
I. Fill in the blanks.
- Hydrogen (H) has only 1 valence electron. So the noble gas closest to it is ____
- One (1) hydrogen atom needs ___ electron to attain the same number of electrons with Helium.
- H2 represents ___ atoms of hydrogen.
- H H shows __ electrons being shared. (how many electrons being shared).
- Fluorine (F) has 7 valence electrons. How many valence electrons it should have to attain stability?
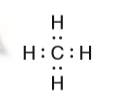
II.Using the structure below, answer the following questions: (The picture is attached below)
- What type of elements are H & C?
- How many electrons are being shared?
- How many electrons does each H share with C?
- Since H has only 1 valence electron, how many valence electrons does C have?
- How many bonds are formed?
Choose 1 element under Group 2, 4 & 6 and draw their Lewis structure. And predict how many electrons are needed by each element to become stable. Write your answer using the following table:
|
Group |
Element |
Lewis structure |
Number of electrons needed to be stable |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Trending now
This is a popular solution!
Step by step
Solved in 2 steps









