A. Ions: Transfer of Electrons 5. Ionic 6. Symbol 7. Name of Ion Element Atomic 2. Electron- 3. Loss or 4. Electron Gain of 1. Electron Number Configuration Charge of Ion Configura- tion of Atom Dot Electrons of Ion Symbol 1s 2s 2p°3s' Na Sodium 11 1+ Na* Sodium lose 1 e Nitrogen 7. Aluminum 13 Chlorine 17 siuoo telu Calcium 20 Охудen 8. B. Ionic Compounds and Formulas abixo (nol abitive (Iraqqo 1. Physical properties Compound Appearance Density Melting Point Sodium chloride, NaCl 2. Formulas of ionic compounds
A. Ions: Transfer of Electrons 5. Ionic 6. Symbol 7. Name of Ion Element Atomic 2. Electron- 3. Loss or 4. Electron Gain of 1. Electron Number Configuration Charge of Ion Configura- tion of Atom Dot Electrons of Ion Symbol 1s 2s 2p°3s' Na Sodium 11 1+ Na* Sodium lose 1 e Nitrogen 7. Aluminum 13 Chlorine 17 siuoo telu Calcium 20 Охудen 8. B. Ionic Compounds and Formulas abixo (nol abitive (Iraqqo 1. Physical properties Compound Appearance Density Melting Point Sodium chloride, NaCl 2. Formulas of ionic compounds
Chapter8: Bonding: General Concepts
Section: Chapter Questions
Problem 26Q: Which of the following statements is(are) true? Correct the false statements. a.The molecules SeS3,...
Related questions
Question
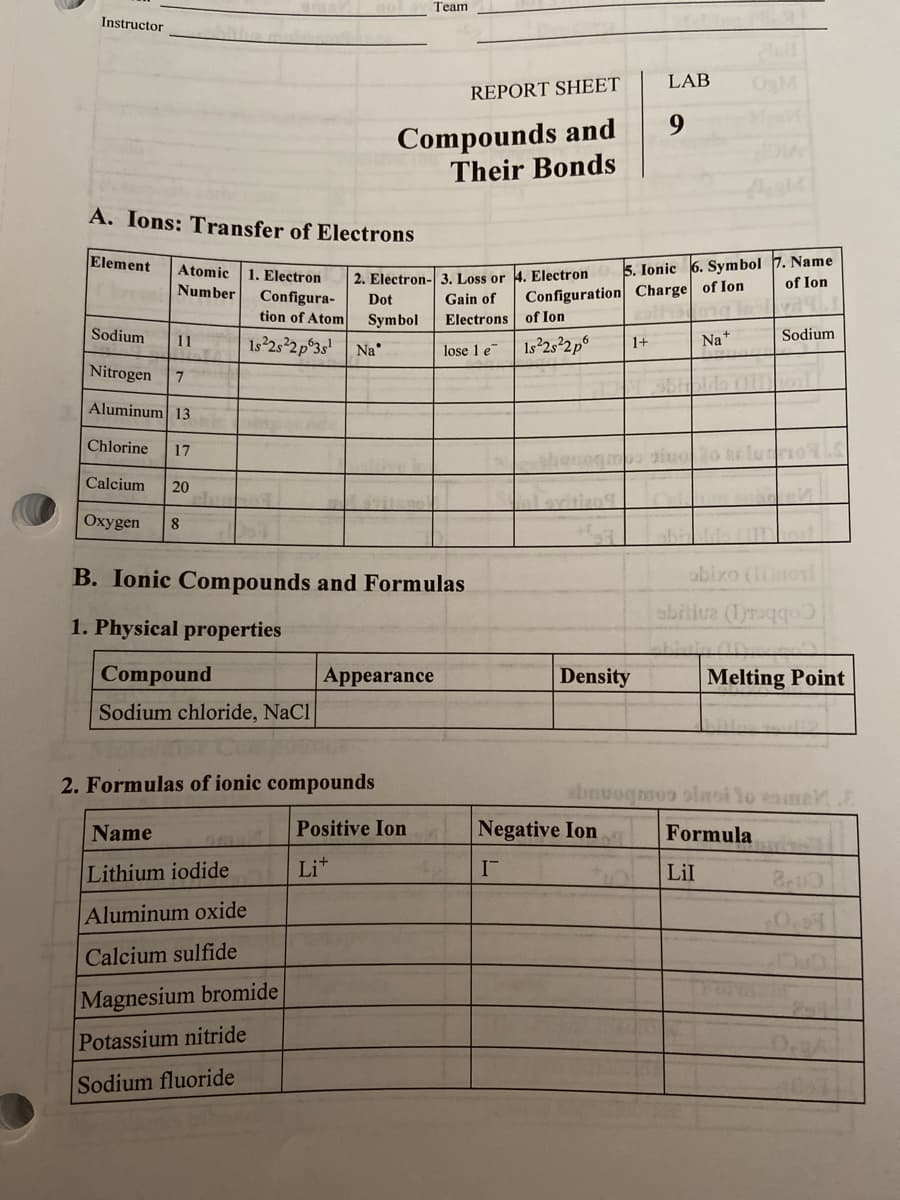
Transcribed Image Text:Team
Instructor
LAB
OgM
REPORT SHEET
Compounds and
Their Bonds
A. Ions: Transfer of Electrons
5. Ionic 6. Symbol 7. Name
of Ion
Element
Atomic
1. Electron
Configura-
2. Electron- 3. Loss or 4. Electron
Number
Dot
Configuration Charge of Ion
Gain of
tion of Atom
Symbol
Electrons of Ion
Sodium
11
Is 252p°3s'
1s 252p6
Na+
Sodium
1+
Na
lose 1 e
Nitrogen
Aluminum 13
Chlorine
17
Calcium
20
evitizo9
Охуgen
8
B. Ionic Compounds and Formulas
obixo (Inonl
sbitive (1raqqo)
1. Physical properties
Compound
Appearance
Density
Melting Point
Sodium chloride, NaCl
2. Formulas of ionic compounds
ebnuoqmos olaoi Yo esme
Name
Positive Ion
Negative Ion
Formula
Lithium iodide
Lit
Lil
Aluminum oxide
Calcium sulfide
Magnesium bromide
Potassium nitride
Sodium fluoride
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning