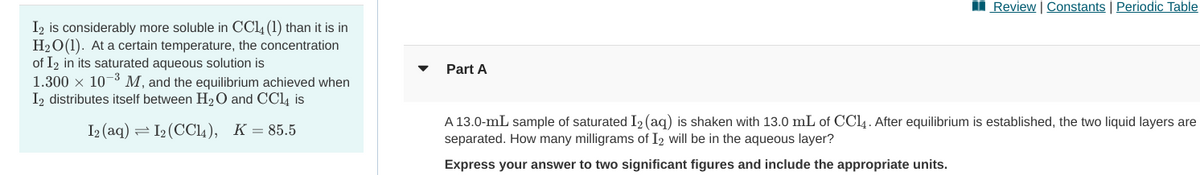
I2 is considerably more soluble in CCL4 (1) than it is in H20(1). At a certain temperature, the concentration of I2 in its saturated aqueous solution is 1.300 x 10-3 M, and the equilibrium achieved when I2 distributes itself between H2O and CCl4 is Part A I2 (aq) = 12 (CC14), K=85.5 A 13.0-mL sample of saturated I2 (aq) is shaken with 13.0 mL of CC14 . After equilibrium is established, the two liquid layers are separated. How many milligrams of I2 will be in the aqueous layer? Express your answer to two significant figures and include the appropriate units.
I2 is considerably more soluble in CCL4 (1) than it is in H20(1). At a certain temperature, the concentration of I2 in its saturated aqueous solution is 1.300 x 10-3 M, and the equilibrium achieved when I2 distributes itself between H2O and CCl4 is Part A I2 (aq) = 12 (CC14), K=85.5 A 13.0-mL sample of saturated I2 (aq) is shaken with 13.0 mL of CC14 . After equilibrium is established, the two liquid layers are separated. How many milligrams of I2 will be in the aqueous layer? Express your answer to two significant figures and include the appropriate units.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter13: Solutions And Their Behavior
Section13.5: Colloids
Problem 2.4ACP
Related questions
Question
Transcribed Image Text:I Review | Constants | Periodic Table
I2 is considerably more soluble in CC4 (1) than it is in
H20(1). At a certain temperature, the concentration
of I2 in its saturated aqueous solution is
1.300 x 10-3 M, and the equilibrium achieved when
I2 distributes itself between H20 and CC4 is
Part A
I2 (aq) = I2 (CC14), K=85.5
A 13.0-mL sample of saturated I2 (aq) is shaken with 13.0 mL of CCl4. After equilibrium is established, the two liquid layers are
separated. How many milligrams of I2 will be in the aqueous layer?
Express your answer to two significant figures and include the appropriate units.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning