Ical Value for k = 7.1 x 10 but was not able to find its unit. Help her find following: 1. Order of the reaction with respect to We (from the graph) 2. Order of the reaction with respect to You (from the graph) 3. Order of the reaction with respect to Nvs (round off to the nearest whole number) 4. Order of the reaction with respect to Luv (round off to the nearest whole number) 5. Unit of the rate constant 6. Rate Law of the reaction 7. [Luv] needed to form 1.0 M of Ae in 5 minutes given that the concentration of the other cor 2.5 M.
Ical Value for k = 7.1 x 10 but was not able to find its unit. Help her find following: 1. Order of the reaction with respect to We (from the graph) 2. Order of the reaction with respect to You (from the graph) 3. Order of the reaction with respect to Nvs (round off to the nearest whole number) 4. Order of the reaction with respect to Luv (round off to the nearest whole number) 5. Unit of the rate constant 6. Rate Law of the reaction 7. [Luv] needed to form 1.0 M of Ae in 5 minutes given that the concentration of the other cor 2.5 M.
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter8: Addition Via Carbocation
Section: Chapter Questions
Problem 18CTQ: The reactants, intermediates, final products, and all curved arrows showing bonds forming...
Related questions
Question
answer 1 to 3
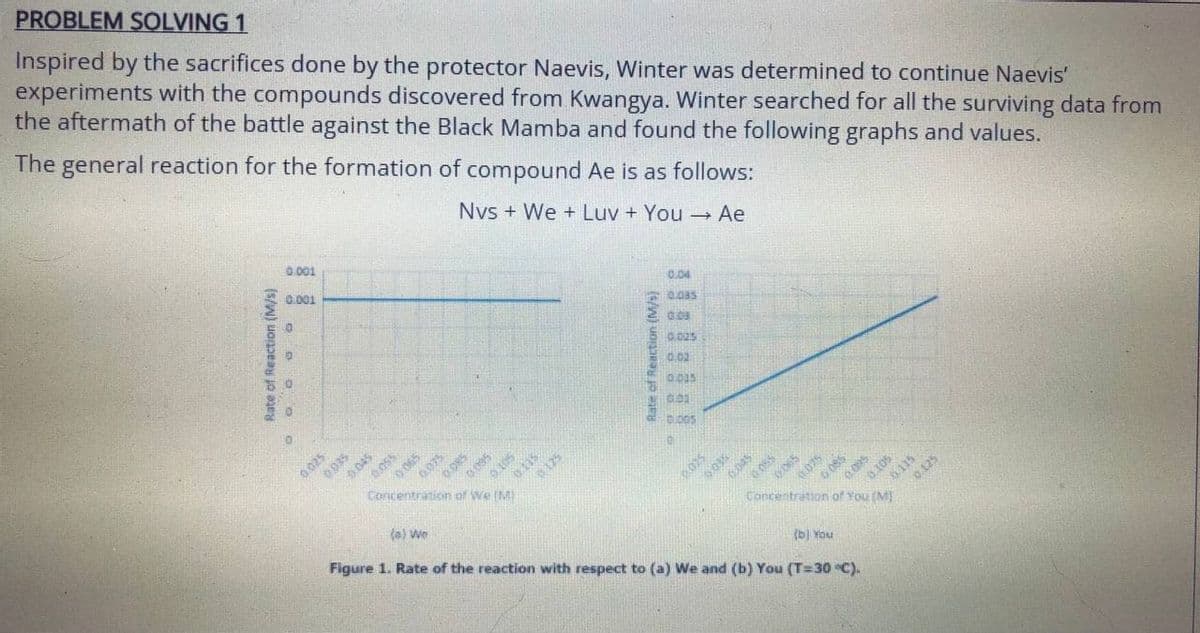
Transcribed Image Text:PROBLEM SOLVING 1
Inspired by the sacrifices done by the protector Naevis, Winter was determined to continue Naevis'
experiments with the compounds discovered from Kwangya. Winter searched for all the surviving data from
the aftermath of the battle against the Black Mamba and found the following graphs and values.
The general reaction for the formation of compound Ae is as follows:
Nvs + We + Luv + You Ae
0.001
0.001
0.04
E 0.035
0.02
0.015
005
0025
0045
Concentration of We IM
Concentration of You (M)
6.115
(a) We
(b) You
Figure 1. Rate of the reaction with respect to (a) We and (b) You (T=30 °C).
Rate of Reaction (M/s)
O o o oo
0065
0.075
0 115
0125
Hate of Reacton (M/s)
0.025
0065
.075
0.095
0.105
0.125
![Table 1. Reaction rates of various Ae formation runs at 30 °C.
Concentration of each component in Solution
Run
[Nvs], M
[We], M
[Luv], M
Initial rate, M/s
[You], M
1
0.053
0.082
0.092
0.046
8.44 x 10-10
2.
0.029
0.096
0.092
0.034
1.87 x 1010
0.053
0.074
0.092
0.034
6.24 x 10-10
She also found a numerical value for k = 7.1 x 105 but was not able to find its unit. Help her find the
following:
1. Order of the reaction with respect to We (from the graph)
2. Order of the reaction with respect to You (from the graph)
3. Order of the reaction with respect to Nvs (round off to the nearest whole number).
4. Order of the reaction with respect to Luv (round off to the nearest whole number)
5. Unit of the rate constant
6. Rate Law of the reaction
7. [Luv] needed to form 1.0 M of Ae in 5 minutes given that the concentration of the other components is
2.5 M.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fb922ac9d-f418-4795-856c-d02abff7b683%2F271bda87-593b-4335-8bb1-3a38c32940d0%2F5hsi3g_processed.jpeg&w=3840&q=75)
Transcribed Image Text:Table 1. Reaction rates of various Ae formation runs at 30 °C.
Concentration of each component in Solution
Run
[Nvs], M
[We], M
[Luv], M
Initial rate, M/s
[You], M
1
0.053
0.082
0.092
0.046
8.44 x 10-10
2.
0.029
0.096
0.092
0.034
1.87 x 1010
0.053
0.074
0.092
0.034
6.24 x 10-10
She also found a numerical value for k = 7.1 x 105 but was not able to find its unit. Help her find the
following:
1. Order of the reaction with respect to We (from the graph)
2. Order of the reaction with respect to You (from the graph)
3. Order of the reaction with respect to Nvs (round off to the nearest whole number).
4. Order of the reaction with respect to Luv (round off to the nearest whole number)
5. Unit of the rate constant
6. Rate Law of the reaction
7. [Luv] needed to form 1.0 M of Ae in 5 minutes given that the concentration of the other components is
2.5 M.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning
