Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter13: Acids And Bases
Section: Chapter Questions
Problem 88QAP: A student is asked to bubble enough ammonia gas through water to make 4.00 L of an aqueous ammonia...
Related questions
Question
100%
Identifique el ácido que tiene la base conjugada más fuerte.
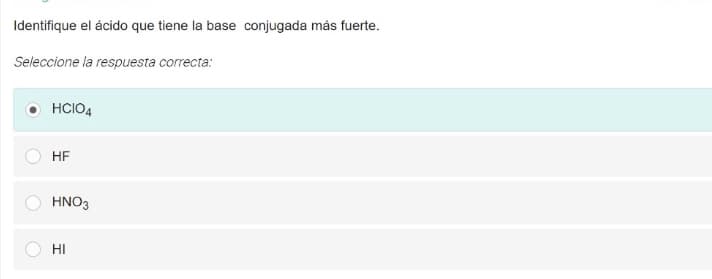
Transcribed Image Text:Identifique el ácido que tiene la base conjugada más fuerte.
Seleccione la respuesta correcta:
HCIO4
HF
HNO3
HI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning
