in a 1.00-cm cell. What is the molar absorptivity at 266 nm? 3.55 x 104 M-1-cm-1 6.54x 104 M-1.cm-1 1.90x105 M-1.cm-1 4.33x105 M-1-cm-1 4.98x105 M-1.cm-1
in a 1.00-cm cell. What is the molar absorptivity at 266 nm? 3.55 x 104 M-1-cm-1 6.54x 104 M-1.cm-1 1.90x105 M-1.cm-1 4.33x105 M-1-cm-1 4.98x105 M-1.cm-1
Chapter24: Introduction To Spectrochemical Methods
Section: Chapter Questions
Problem 24.2QAP
Related questions
Question
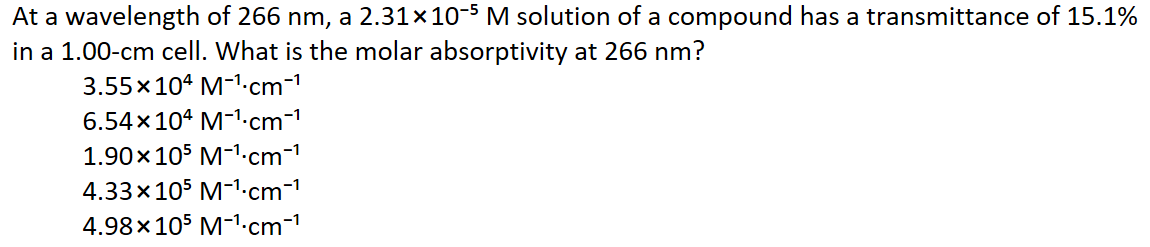
Transcribed Image Text:At a wavelength of 266 nm, a 2.31×10-5 M solution of a compound has a transmittance of 15.1%
in a 1.00-cm cell. What is the molar absorptivity at 266 nm?
3.55x104 M-1.cm-1
6.54x 104 M-1-cm-1
1.90x105 M-1.cm-1
4.33x105 M-1.cm-1
4.98x 105 M-1.cm-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning