Identify if the alkene has been oxidized, reduced or neither. (Hint: first look at each carbon atom separately and then look at the net charge for the alkene as a whole)
Identify if the alkene has been oxidized, reduced or neither. (Hint: first look at each carbon atom separately and then look at the net charge for the alkene as a whole)
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter13: Alcohols, Phenols, And Ethers
Section: Chapter Questions
Problem 13.59E
Related questions
Question
Identify if the alkene has been oxidized, reduced or neither. (Hint: first look at each carbon atom separately and then look at the net charge for the alkene as a whole)
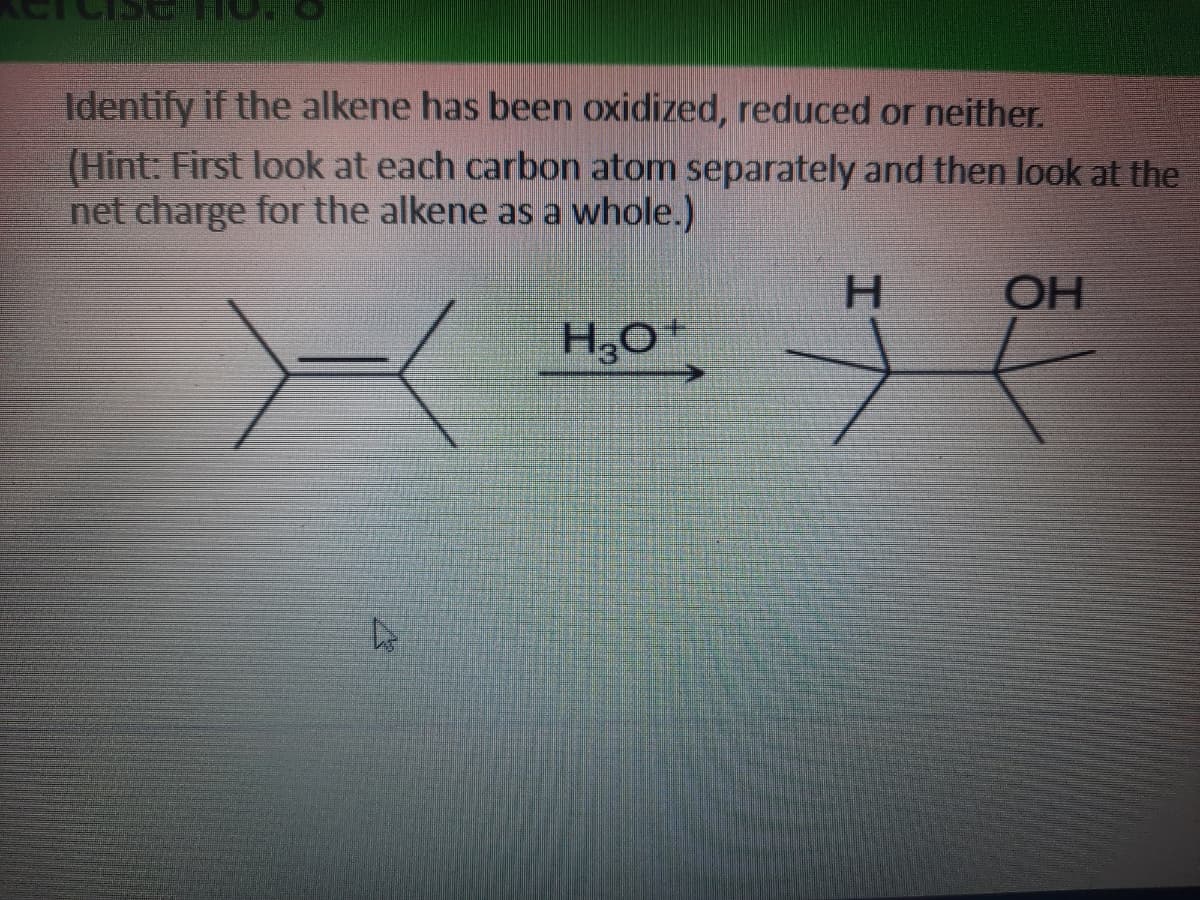
Transcribed Image Text:Identify if the alkene has been oxidized, reduced or neither.
(Hint: First look at each carbon atom separately and then look at the
net charge for the alkene as a whole.)
H.
OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning