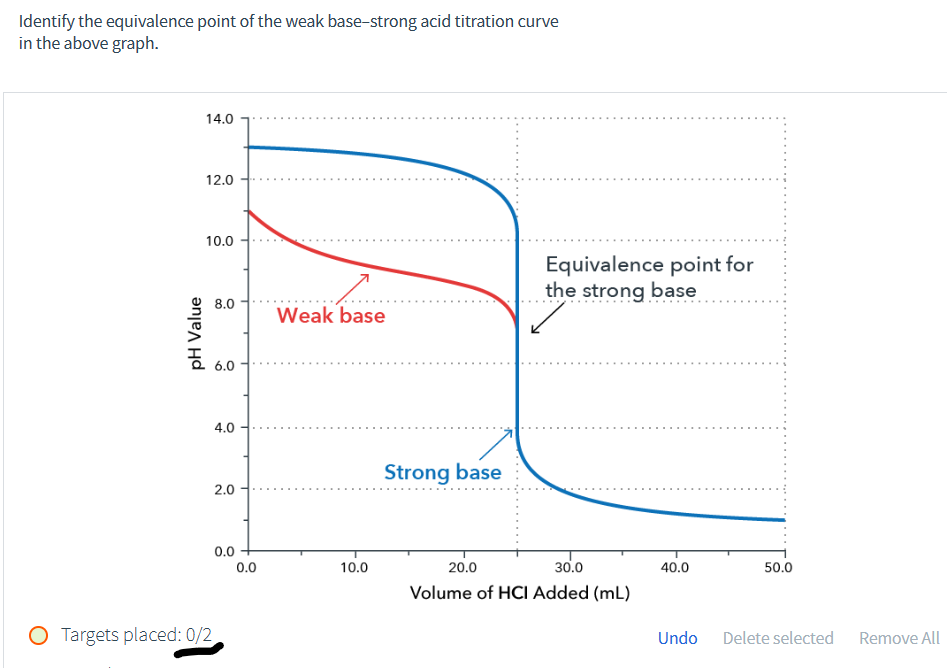
Identify the equivalence point of the weak base-strong acid titration curve in the above graph. pH Value 14.0 12.0 10.0 8.0 Weak base 6.0 4.0 2.0 0.0 0.0 10.0 Strong base 20.0 Equivalence point for the strong base 30.0 Volume of HCI Added (mL) 40.0 50.0
Q: + CI 1) FeCl3 2) H₂O ?
A: The objective of this question is to determine the product of the reaction between iron(III)…
Q: Sketch the shape and orientation of the following types of orbitals. a. px b. dyz
A: There is need to find shape and orientation of Px and dyz orbitals .
Q: Predict the major product of the following reaction. 1. CH3MgBr (2 eq.) 2. H3O+ Draw the molecule on…
A: An arrow always depicts from a region of high electron density to low electron density ; that is…
Q: What quantity in moles of sodium atoms do you have if you have 2.10 × 10²³ atoms of sodium. (The…
A: The objective of the question is to find out the quantity in moles of sodium atoms given the number…
Q: Some soluble compounds are listed in the table below. Complete the table by filling in the name or…
A: Part I.Part II.Explanation:Part I.Ionic compounds consist of positive (metal) and negative ions…
Q: A chemist was charged with the task of making amino alcohol 2. This chemist decided to attempt a…
A: The objective of the question is to identify the structures of the two products formed in the…
Q: Determine the mass, in grams, of 0.785 moles of Si (1 mol of Si has a mass of 28.09 g)
A: The number of molecules or atoms present in the one mole of the substance is equal to the Avogadro…
Q: Determine the energy change associated with the transition from n=3 to n=2 in the hydrogen atom. OA.…
A:
Q: Determine the Kovats retention index for an unknown C9 sample using the retention times 1.3 min for…
A: Calculate retention index for an unknown C9 sample.
Q: Draw the major product of this reaction. Ignore inorganic byproducts. + NH2NH2, KOH heat Drawing Q
A:
Q: MISSED THIS? Watch KCV: Buffers; Read Section 18.2. You can click on the Review link to access the…
A: A buffer is a solution which resists any change in pH on adding a small amount of acid or base.It is…
Q: From the following reactive carbon intermediates, please select which you would expect to be the…
A: To solve this problem we have to identify the most stable carbocation intermediate.
Q: A. For each reaction 1) Provide the missing reagents/conditions or major organic products as…
A: The objective of this question is to identify appropriate reagents and conditions for specific…
Q: Draw the major product of this reaction. Ignore inorganic byproducts. ssume that the water side…
A: The objective of the question is to predict the product formed in the given reaction.
Q: Do the reactions below proceed in good yield from left to right as shown? b) excess OH c) OH H₂O да…
A: The objective of the question is to predict the reactions that give good yield from the given…
Q: A diprotic acid, H2A, has acid dissociation constants of Kal = 3.78 x 104 and Ka2 = 4.21 x 10-11.…
A: A diprotic acid can dissociate to produce two H+ ions.
Q: Write the molecular formula of acetic acid. The color codes for the ball-and-stick model shown are:…
A: Here, in the structure, black color shows carbon, blue red shows oxygen, and white shows hydrogen…
Q: Question 1: Show the major products, with stereochemistry where applicable, for the reactions of…
A: The objective of the question is to predict the major products of the reactions of 1-pentyne with…
Q: The given structure is Bonducellin. It's Molecular weight is 282. Perform fragmentation to this…
A: Mass spectrometry is mainly used for the identification of molecular weight and molecular formula.…
Q: 7. Which of the following carbocations is least likely to undergo a 1,2-rearrangement? Bubble in…
A: Carbocation can be defined as the species in which a carbon atom carries positive charge. In the…
Q: HO. Na2Cr2O7 H2SO4, H₂O SOCI₂ OH H3O+ 1. LIAI(OMe)3H 2. H3O+ excess NH3
A: The reaction scheme is shown below.We have to provide the missing products and reagents.
Q: 1.0 L of a buffer solut What is the pH after 0 O a. 9.928 O b.9.602 O c. 9.319 O d. 9.193 Oe. 9.398
A:
Q: What term best describes the Tation below? COOH H3C-CH COOH соон HC-CH3 OH HO
A: The molecules with the same molecular formula but different structures are known as isomers. If the…
Q: Using the simulator below identify the base from the options shown.
A: The objective of the question is to identify bases based on given values to recognize substances…
Q: Predict the product of this organic reaction: OH [0] P+H2₂O Specifically, in the drawing area below…
A: The oxidation of alcohol depends on the number of hydrogen atoms attached to the carbon atom that…
Q: on or neithe Classify the transformation below as oxidation, (b) Please identify the oxidation state…
A: In chemistry, oxidation refers to an increase in the oxidation state, and reduction means a decrease…
Q: 10.0 mL sample of an HCl solution is titrated with a 1.00 M NaOH solution. The neutralization…
A: Volume of HCl solution= 10 mL Molarity of NaOH= 1.0 M Volume of NaOH solution= 2.45 mL Molarity is…
Q: In a saturated solution that is in contact with solid Rb(ClO4)2, the concentration of Rb is 0.0361…
A: Rb(ClO4)2(s) ⇌ Rb2+(aq) + 2ClO4-(aq)Concentration of Rb2+ = [Rb2+] = s = 0.0361 MSolubility product…
Q: Problem 6 of 20 Draw an alkyl halide that would undergo an SN2 reaction to yield this product under…
A: Final answer is given in explanation please see from there.Explanation:Approach to solving the…
Q: For the reaction below, Kc = 1.10 x 10-8. Note Kc is sometimes called What is the equilibrium…
A: Equilibrium constant is defined as the ratio of concentration of products to the concentration of…
Q: 3. A sample of neon effuses from a container in 76 seconds. The same amount of an unknown noble gas…
A: The concept of Graham's law of effusion is used here in which the ratio of the rate of effusion is…
Q: Which mineral(s) is a sheet silicate? pyroxene biotite ☐ amphibole muscovite quartz
A: Silicates are minerals made up of silicon and oxygen arranged in tetrahedral SiO44- Units that are…
Q: 2. Complete the following reaction. Show the intermediate, and propose a mechanism for the second…
A: This is an example of Hoffmann elimination reaction. In this reaction proton is abstracted from the…
Q: Question 1: Show the major products, with stereochemistry where applicable, for the reactions of…
A: The objective of the question is to predict the major products of the reactions of 1-pentyne with…
Q: 12. What is the expected major product for the following reaction? HO HCI (g) CH3OH
A: Carbonyl compounds (aldehyde and ketone) react with an alcohol to form hemiacetal in the presence of…
Q: Type in your numeric answer showing two decimal places to identify the pH of a solution with the…
A: Given that,The concentration of hydronium ion,(Assume unit in M)pH of the solution= ?
Q: Determine the pH during the titration of 58.3 mL of 0.310 M acetic acid (K, = 1.8×10) by 0.310 M…
A:
Q: Energy Part 1 of 2 5x 4d Condensed electron configuration: Part 2 of 2 Group number: X Sp × Give…
A: Answer:Distribution of electrons in a multi- electron system using afbau principle is called its…
Q: Show three or four different drawings that show three or four different relevant resonance…
A: Resonance structures are the different structures that represent the delocalization of electrons in…
Q: Which element(s) would you expect to find in box #6? 1 0 0 Si Si 2 - 0 0 0 0 0. Si Si 0 0 0 0 0 6 3…
A: In the the given structure we have to identify which atom will occupy the place of box #6
Q: N2O(g)+ H2O(g) 2NO(g) + H2(g) Initial cone's N2O H2O Initial rate of…
A: The objective of the question is to determine the rate expression, the overall order of the…
Q: H3CO OH 2 HCONH2
A: The Leuckart reaction involves the reaction between a carbonyl compound and a formamide. Ammonium…
Q: Q2. A) Determine the monomer unit of the following polymer CH3 CH3 CH3 CH₂-C-CH2-C-CH2-7 CO2CH3…
A: A monomer is a small molecule that can chemically bond to other monomers to form a polymer. Monomers…
Q: Determine the minimum concentration of the precipitating agent on the right to cause precipitation…
A:
Q: (B) Identify the IUPAC name for the following compound. Br. OH A.…
A: Alkene show two stereochemistry :-Z indicates that the higher priority substituents are on the same…
Q: Select all of the reagents from the list that can be used to accomplish the transformation shown…
A: The process during which the addition of H2 molecules occurs across an unsaturated double or triple…
Q: Provide all reasonable resonance structures for each molecular structure below. Use curved arrows to…
A: These are the collection of Lewis structures which represents the delocalisation of electrons in a…
Q: Draw a structural formula for the product of the reaction shown. CH3 NC CO₂CH3 H H CH3
A: The objective of the question is to identify the product.
Q: Give the major product of the following reaction. ?
A:
Q: Calculate the mass of 8.70 × 10³ mol of Mg(NO3)2 . Round your answer to 3 significant figures.
A: The unit for mass is grams (g) and molar mass is g/mol. To get the mass, the moles have to be…
Identify the equivalence point of the weak base–strong acid titration curve in the graph.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

- Advantages of potentiometric titrations over 'classical' visual indicator methods: (select the correct statement(s)). Can be used for colored, turbid or fluorescent analyte solution. Can be used if there is no suitable indicator or if the color change is difficult to visualize. Both answers 1 and 2 are correctSources of Error Determine the relationship between the observed/apparent value (EX) VERSUS that of the true value (ET) for the quantity being sought by writing either <, >, or = on the space provided TOPIC: Determination of Molar Concentration of each component (Double Indicator Titration)1. No blank correction Ex _______ET2. Bubbles trapped in the tip of burette: EX ______ ET3. Measuring the sample volume using a volumetric pipet while looking downwards at the meniscus: EX _____ ET2,5 ml volume has taken from an “hypothetic” solution which includes (3+) Sb and (3+) Fe and at the titration with 0.1004 N KMnO4, the wasted amount has found as 16,4 mL. The other 2,5 mL that has taken, has reduced with Zn after that, this 2,5 mL solution has titrates with the same KMnO4 solution solution and the wasted amount is 26,5mL. With these datas find the %concentrations of the ions at the solution.
- A RbOH solution is titrated four (4) times against potassium hydrogen phthalate (KHP; FW=204.224) samples to the Phenolphthalein endpoint. Using the data below, determine the concentration of the RbOH solution? g of KHP Volume of Base Required 0.5373 g 42.49 mL 0.5856 g 43.88 mL 0.5790 g 48.56 mL 0.5856 g 44.60 mL (Report your answer as "mean +/- std dev") M What is the percent relative standard deviation? % What is the 99% Confidence Interval for the concentration of the solution (population mean)?A 250.0mg sample of an organic monoprotic weak acid was dissolved in an appropriate solvent and titrated with 0.091M NAOH , requiring 29.5ml to reach end point. Determine equivalent weightplease stop rejecting. we pay for this service, so we deserve getting our answer! I do not want to have to put a claim in and report Use acid-base titration to determine theconcentration of:– A strong acid: HCl 0.100 M NaOH (standardized)•0.100 M HCl0.100 M CH3COOH Phenolphthalein is used for endpoint determination– Changes from colourless to pink as a solution becomes more basic• Only need 2-3 drops per titration• pH range: 8.3-10
- 1) The equivalence points of the two titrations curves were not in the same pH range explain why. 2) Using the experimental data at 1/2 the equivalence point volume, calculate the Ka of acetic acid. Volume of NaOH at Equivalence point for HCl Trial = 5.083ml Equivalence point pH for HCl Trial = 6.57 pH 1/2 Equivalence point for HCl Trial = 3.285 Volume of NaOH at Equivalence point for HC2H3O2 = 4.025ml Equivalence point pH for HC2H3O2 Trial = 4.13 pH 1/2 Equivalence point for HC2H3O2 Trial = 2.065While preparing the samples for analysis, you forgot that you already added phenolphthalein indicator so you added some more. What will be its effect in the volume of the titrant needed to reach the endpoint? Select one: A. Increase B. Decrease C. No effect D. Cannot be determined Primary standards are used to determine the exact concentration of the titrant. Based on the criteria of primary standards, which of the following cannot be used as a primary standard? Select one: A.Oxalic acid B. Sodium hydroxide C. Sodium carbonate D. Potassium dichromate During titration, you notice that the retention of the faint pink color is longer than earlier. Your lab partner suggested that you add a “half-drop” to prevent over-titration of the analyte solution. Is it ethical to do the “half-drop” technique? Select one: A. NO. It will cause error in calculations if you forgot to record the volume reading before adding the half-drop. B. YES. No error in calculations will be encountered provided…Using the derived endpoints for both phosphoric acid titrations, calculate theconcentration of the phosphoric acid solution and report the mean value. Vol Mid Point 1st Derivative 3.96 0.089821268 5.91 0.229138878 7.105 0.257152358 8.345 0.562805368 9.025 1.958394009 9.175 4.406074475 9.215 10.52631579 9.27 5.043426253 9.355 4.129394633 9.415 7.738714375 9.465 2.260096883 9.505 13.04347826 9.605 1.258033788 9.8 0.85812357 10 0.845641773 10.25 0.636571666 10.65 0.415019763 11.2 0.31048471 11.95 0.277373275 12.925 0.270438943 13.875 0.273865319 14.55 0.391304348 15.025 0.421607378 15.425 0.567030224 15.7 0.934574579 15.9 0.898689411 16.05 1.831703765 16.125 4.911587269 16.175 4.137715831 16.25 3.061160807 16.325 7.932182234 16.375 6.403162055 16.45 3.070522155 16.55 1.981485334 16.675 1.751612232 16.875 0.8562513 17.075 1.352194716 17.375 0.427848277 17.85 0.434990639 18.475 0.249635948 19.275…
- Chemistry A 20 mL solution containing both Ca2+ and Mg2+ cations is diluted in to 100 mL. When 10 mL of this solution is taken and titrated with 0.05 M EDTA at pH = 10 in the presence of Erio – T indicator, the consumption is found as 12 mL. A new 10 mL was taken from the same solution and (NH4)2C2O4 is added on it and then formed the precipitate is filtered. The filtered solution is titrated with the same EDTA solution and the consumption is found as 3 mL. So find the Ca2+ and Mg2+ amounts in the main sample solution in terms of mg/L.Sources of Error Determine the relationship between the observed/apparent value (EX) VERSUS that of the true value (ET) for the quantity being sought by writing either <, >, or = on the space provided TOPIC: Standardization of Titrant Question 7 Distilled water was not equilibrated to room temperature before the preparation of NaOH titrant. EX __ ET TOPIC: Determination of Molar Concentration of each component (Double Indicator Titration) Question 8 No blank correction Ex ___ ET Question 9 Bubbles trapped in the tip of burette: EX ___ ET Question 10 Measuring the sample volume using a volumetric pipet while looking downwards at the meniscus: EX ___ ETIm doing a lab for chemistry and need help answering numers 1 and 2 below using the data provided. The molar concentration of the sodium for both is .100M. For your coarse titration, record the following results. initial burette volume reading of NaOH titrant at the start (mL) 0.00 final burette volume reading of NaOH titrant before the color change (mL) 29.05 volume of NaOH solution dispensed (mL) 29.05 volume of HCl solution in the flask (mL) 5.00 1) Calculate the molar HCl concentration using your coarse titration results. For your fine titration, record the following results. initial burette volume reading of NaOH titrant at the start (mL) 0.00 burette volume reading of NaOH titrant at the color change (mL) 30.05 volume of NaOH solution dispensed (mL) 30.05 volume of HCl solution in the flask (mL) 5.00 2)Calculate the molar HCl concentration using your fine titration results.



