Identify the following reactions as formation, simple decomposition or other. Reaction 2H₂O₂(1) 2H₂O(1) + O₂(g) NH3(g) + H₂O(l) NH4OH(aq) Fe(s) + O₂(g) → FeO(s) HF(g) - H₂(g) + F2(9) Classification
Identify the following reactions as formation, simple decomposition or other. Reaction 2H₂O₂(1) 2H₂O(1) + O₂(g) NH3(g) + H₂O(l) NH4OH(aq) Fe(s) + O₂(g) → FeO(s) HF(g) - H₂(g) + F2(9) Classification
Chapter9: Energy For Today
Section: Chapter Questions
Problem 11E: Define each of the following terms: a. heat b. energy c. work d. system e. surroundings f....
Related questions
Question
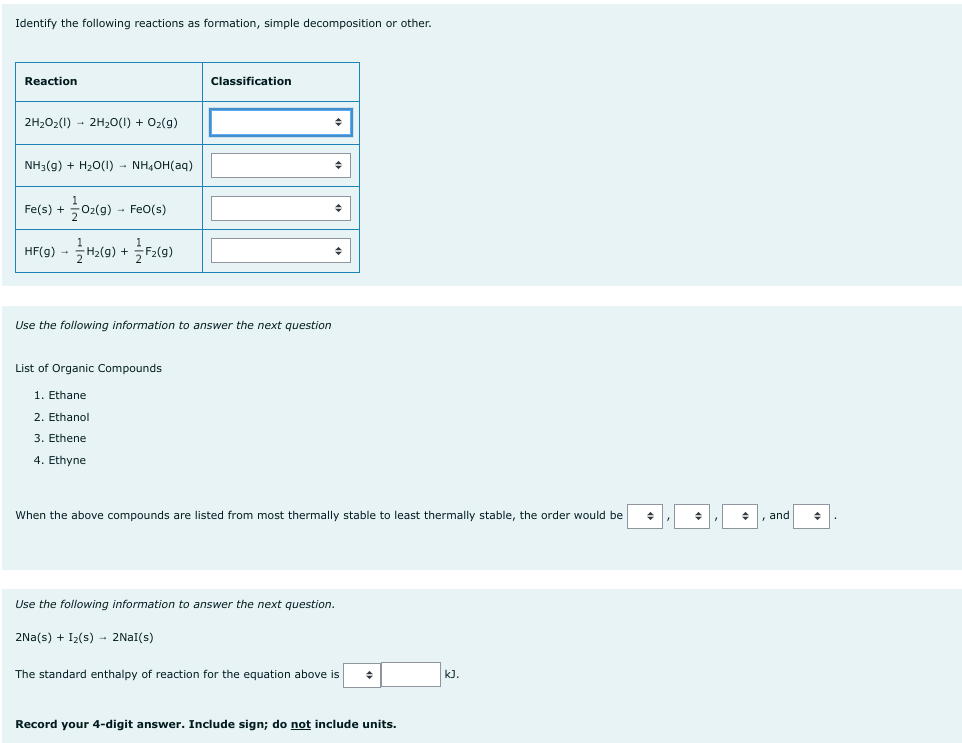
Transcribed Image Text:Identify the following reactions as formation, simple decomposition or other.
Reaction
2H₂O₂(1)→ 2H₂O(l) + O₂(g)
NH3(g) + H₂O(1) - NH₂OH(aq)
+0₂(g) → Fe0(s)
HF(g) →
- H₂(g) + F2(g)
Fe(s) +
Use the following information to answer the next question
List of Organic Compounds
Classification
1. Ethane
2. Ethanol
3. Ethene
4. Ethyne.
+
+
When the above compounds are listed from most thermally stable to least thermally stable, the order would be
Use the following information to answer the next question.
2Na(s) + I₂(s)→ 2Nal(s)
The standard enthalpy of reaction for the equation above is
+
Record your 4-digit answer. Include sign; do not include units.
kJ.
+
and
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning