Identify the species oxidized, the species reduced, the oxidizing agent and the reducing agent in the following electron-transfer reaction. Pb(s) + 2FE3*(aq) - → Pb²*(aq) – 2Fe²*(aq) species oxidized species reduced oxidizing agent reducing agent As the reaction proceeds, electrons are transferred from to
Identify the species oxidized, the species reduced, the oxidizing agent and the reducing agent in the following electron-transfer reaction. Pb(s) + 2FE3*(aq) - → Pb²*(aq) – 2Fe²*(aq) species oxidized species reduced oxidizing agent reducing agent As the reaction proceeds, electrons are transferred from to
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter19: The Chemistry Of The Main-group Elements
Section19.6: A Periodic Perspective: The Main-group Elements
Problem 19.8PSP
Related questions
Question
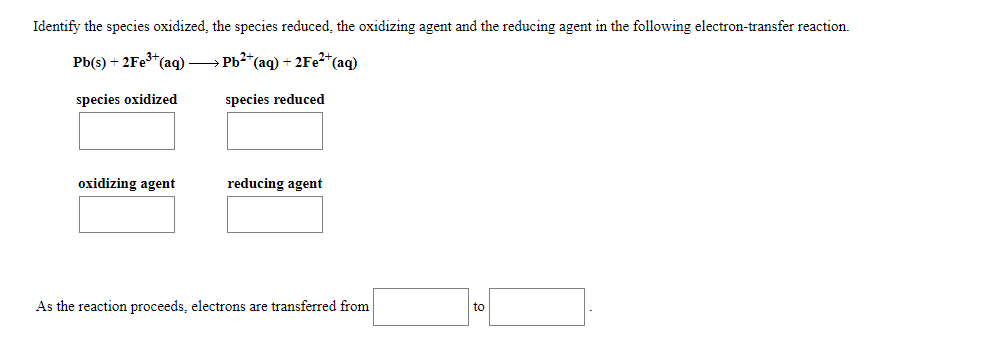
Transcribed Image Text:Identify the species oxidized, the species reduced, the oxidizing agent and the reducing agent in the following electron-transfer reaction.
Pb(s) + 2FE3*(ag) → Pb2*(aq) + 2F22*(aq)
species oxidized
species reduced
oxidizing agent
reducing agent
As the reaction proceeds, electrons are transferred from
to
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning