Identify the strongest attractive force present in the pure substances shown: London forces, dipole-dipole, hydrogen bonding, ion-dipole, or ionic attractions. Drag the appropriate items to their respective bins. Reset Help KCI CH3CH2CH2NH2 CH3OCH2CH3 CH2Cl2 Dipole-dipole attractions Hydrogen bonding lon-dipole attractions lonic attractions London forces
Identify the strongest attractive force present in the pure substances shown: London forces, dipole-dipole, hydrogen bonding, ion-dipole, or ionic attractions. Drag the appropriate items to their respective bins. Reset Help KCI CH3CH2CH2NH2 CH3OCH2CH3 CH2Cl2 Dipole-dipole attractions Hydrogen bonding lon-dipole attractions lonic attractions London forces
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter15: Gases,liquids, And Solids
Section: Chapter Questions
Problem 15.2TC
Related questions
Question
Solve it asap..
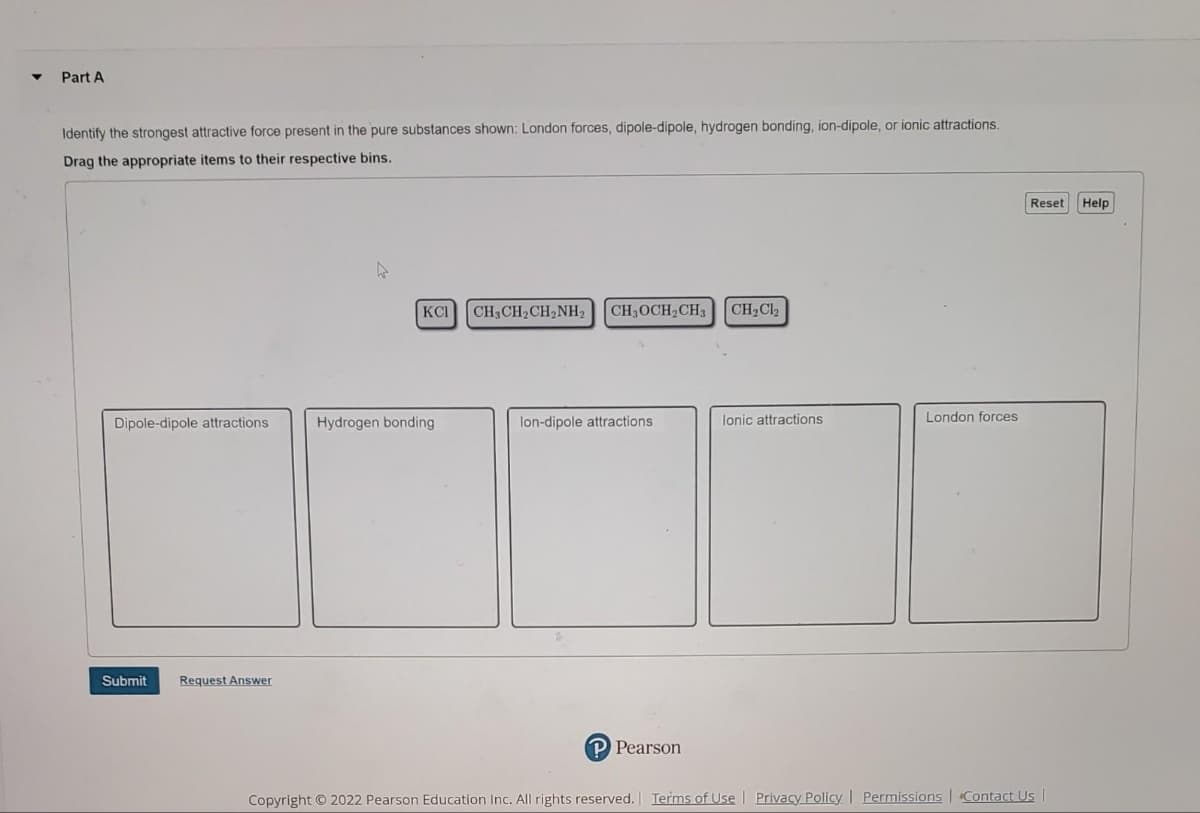
Transcribed Image Text:Part A
Identify the strongest attractive force present in the pure substances shown: London forces, dipole-dipole, hydrogen bonding, ion-dipole, or ionic attractions.
Drag the appropriate items to their respective bins.
Reset Help
KCI
CH3OCH2CH3
CH2Cl2
Dipole-dipole attractions
Hydrogen bonding
lon-dipole attractions
lonic attractions
London forces
Submit
Request Answer
Pearson
Copyright © 2022 Pearson Education Inc. All rights reserved. Terms of Usel Privacy Policy I Permissions | Contact Us |
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning