The equilibrium reaction for the ionization of aspartate from a low to a high pH is shown below. / Die ewewigsreaksie vir die lonisasie var aspartiensuur vanaf 'n lae na 'n hoë pH word hieronder getoon. COOM COOK COOH COOH CH CH, COOH COOH COOH COOH pK, values/pk, waardes. a-COOH - 2.2; a-NH, - 9.1; B-COOH - 3.9 At what pH are there equal amounts of C and D?/ By watter pH is daar gelyke hoeveelhede van C en D? 4.6 B. 9.1 10.6 6.5
The equilibrium reaction for the ionization of aspartate from a low to a high pH is shown below. / Die ewewigsreaksie vir die lonisasie var aspartiensuur vanaf 'n lae na 'n hoë pH word hieronder getoon. COOM COOK COOH COOH CH CH, COOH COOH COOH COOH pK, values/pk, waardes. a-COOH - 2.2; a-NH, - 9.1; B-COOH - 3.9 At what pH are there equal amounts of C and D?/ By watter pH is daar gelyke hoeveelhede van C en D? 4.6 B. 9.1 10.6 6.5
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter23: Organic Polymers, Natural And Synthetic
Section: Chapter Questions
Problem 46QAP: Glycolysis is the process by which glucose is metabolized to lactic acid according to the equation...
Related questions
Question
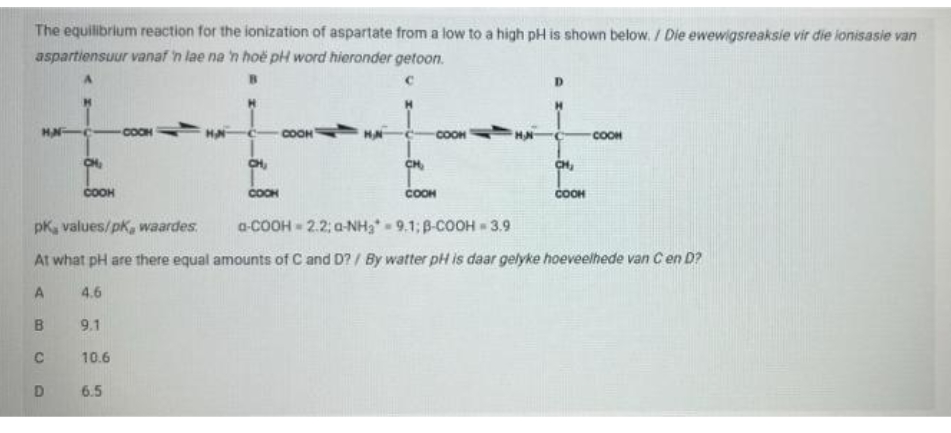
Transcribed Image Text:The equilibrium reaction for the ionization of aspartate from a low to a high pH is shown below. / Die ewewigsreaksie vir die ionisasie van
aspartiensuur vanaf 'n lae na 'n hoë pH word hieronder getoon.
HAN
COOH
HA
COOH
COOH
CH
COOH
COOH
COOH
COOH
pK, values/pk, waardes.
a-COOH 2.2; a-NH3 9.1; B-COOH 3.9
At what pH are there equal amounts of C and D? / By watter pH is daar gelyke hoeveelhede van Cen D?
4.6
B.
9.1
C
10.6
D.
6.5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning