If 58 moles of NH 3 are combined with 32 moles of sulfuric acid, what is the limiting reactant and how much of the excess reactant is left over? 2NH 3 + H 25O 4→ (NH 4) 25O 4 O a. H2SO4, 3.0 mol O b. NH3, 1.0 mol O c. NH3, 3.0 mol O d. H2SO4, 29 mol O e. NH3, 29 mol
If 58 moles of NH 3 are combined with 32 moles of sulfuric acid, what is the limiting reactant and how much of the excess reactant is left over? 2NH 3 + H 25O 4→ (NH 4) 25O 4 O a. H2SO4, 3.0 mol O b. NH3, 1.0 mol O c. NH3, 3.0 mol O d. H2SO4, 29 mol O e. NH3, 29 mol
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter4: Stoichiometry
Section: Chapter Questions
Problem 4.9PAE: 4.9 Sulfur, S8, combines with oxygen at elevated temperatures to form sulfur dioxide. (a) Write a...
Related questions
Question
9 This is for my reviewer please help me with the step by step solution and answer, thank you
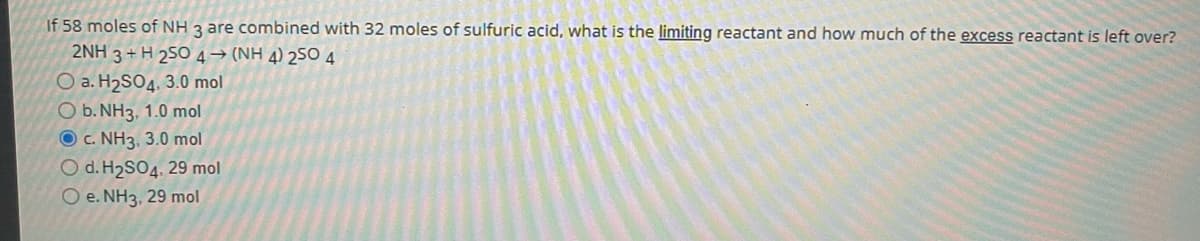
Transcribed Image Text:If 58 moles of NH 3 are combined with 32 moles of sulfuric acid, what is the limiting reactant and how much of the excess reactant is left over?
2NH 3 + H 25O 4→ (NH 4) 25O 4
O a. H2SO4, 3.0 mol
O b. NH3, 1.0 mol
O c. NH3, 3.0 mol
O d. H2SO4, 29 mol
O e. NH3, 29 mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning