If you have 155 mL solution of a 0.762 M FeCl3 solution, how many moles of FeCl3 are contained in this sample? X STARTING AMOUNT RESET ADD FACTOR ANSWER
If you have 155 mL solution of a 0.762 M FeCl3 solution, how many moles of FeCl3 are contained in this sample? X STARTING AMOUNT RESET ADD FACTOR ANSWER
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter13: The Chemistry Of Solutes And Solutions
Section: Chapter Questions
Problem 47QRT
Related questions
Question
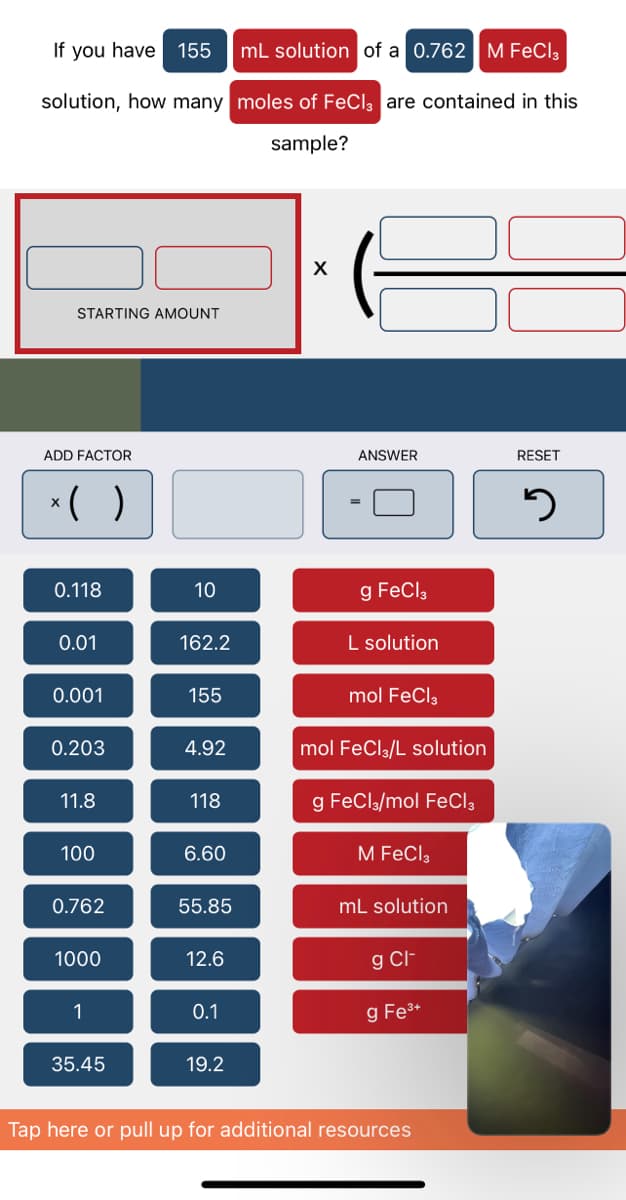
Transcribed Image Text:If you have 155 mL solution of a 0.762 M FeCl3
solution, how many
moles of FeCl3 are contained in this
sample?
X
STARTING AMOUNT
RESET
3
ADD FACTOR
ANSWER
0.118
10
g FeCl3
0.01
162.2
L solution
0.001
155
mol FeCl3
0.203
4.92
mol FeCl3/L solution
11.8
118
g FeCl3/mol FeCl3
100
6.60
M FeCl3
0.762
55.85
mL solution
1000
12.6
g Cl-
1
0.1
g Fe³+
35.45
19.2
Tap here or pull up for additional resources
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning