(ii) Explain one reason for using aluminium in making electrical cables and drink cans respectively. Aircraft bodies.. Drink cans... (f) Sulfur trioxide reacts with water to form sulfuric acid. Sulfuric acid reacts with metals to form a solution of sulfate salt, for example zinc forming zinc sulfate solution. Explain why a piece of lead stops reacting soon after being added to sulfuric acid even though it can react with sulfuric acid.
(ii) Explain one reason for using aluminium in making electrical cables and drink cans respectively. Aircraft bodies.. Drink cans... (f) Sulfur trioxide reacts with water to form sulfuric acid. Sulfuric acid reacts with metals to form a solution of sulfate salt, for example zinc forming zinc sulfate solution. Explain why a piece of lead stops reacting soon after being added to sulfuric acid even though it can react with sulfuric acid.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter4: Reactions In Aqueous Solution
Section: Chapter Questions
Problem 74QAP: A solution contains both iron(II) and iron(III) ions. A sample Of the solution is titrated with 35.0...
Related questions
Question
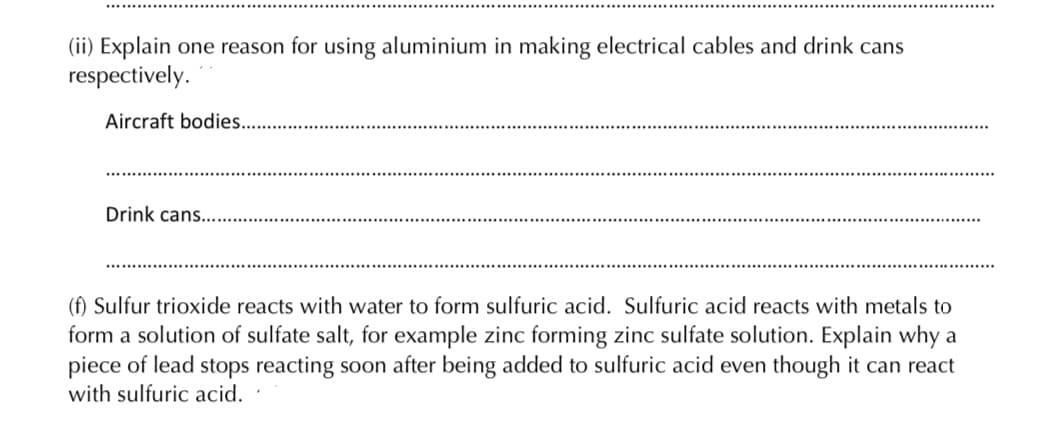
Transcribed Image Text:(ii) Explain one reason for using aluminium in making electrical cables and drink cans
respectively.
Aircraft bodies..
Drink cans...
(f) Sulfur trioxide reacts with water to form sulfuric acid. Sulfuric acid reacts with metals to
form a solution of sulfate salt, for example zinc forming zinc sulfate solution. Explain why a
piece of lead stops reacting soon after being added to sulfuric acid even though it can react
with sulfuric acid.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning