Imagine you have a water bottle that is partially filled with water and sealed. Which situation means that the system has reached equilibrium? O The rate of condensation is greater than the rate of evaporation. O The rate of evaporation is zero. O The rate of evaporation is greater than the rate of condensation. O The rate of evaporation is equal to the rate of condensation.
Imagine you have a water bottle that is partially filled with water and sealed. Which situation means that the system has reached equilibrium? O The rate of condensation is greater than the rate of evaporation. O The rate of evaporation is zero. O The rate of evaporation is greater than the rate of condensation. O The rate of evaporation is equal to the rate of condensation.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter17: Equilibrium
Section: Chapter Questions
Problem 87AP: . Many sugars undergo a process called mutarotation, in which the sugar molecules interconvert...
Related questions
Question
100%
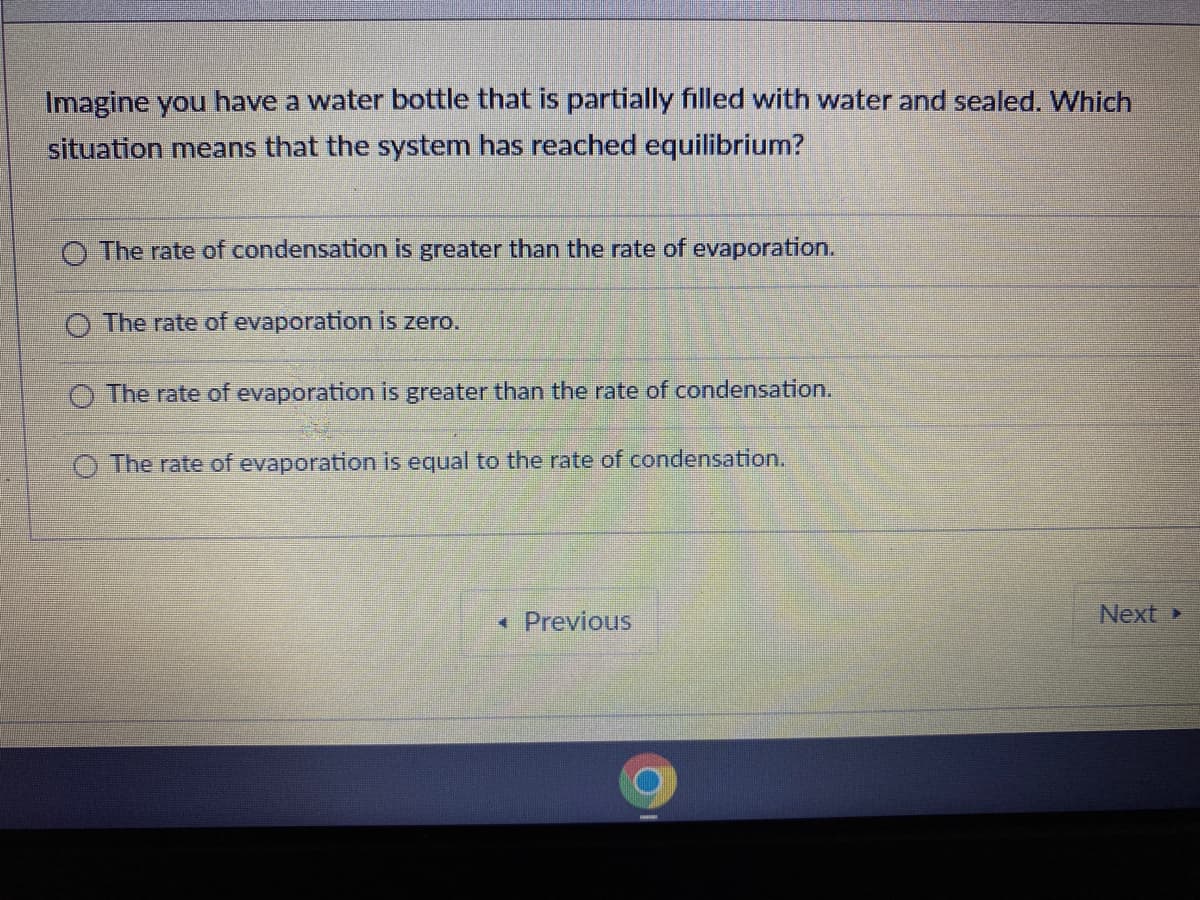
Transcribed Image Text:Imagine you have a water bottle that is partially filled with water and sealed. Which
situation means that the system has reached equilibrium?
O The rate of condensation is greater than the rate of evaporation.
O The rate of evaporation is zero.
O The rate of evaporation is greater than the rate of condensation.
O The rate of evaporation is equal to the rate of condensation.
• Previous
Next>
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning