In 1887, the Russian chemist Sergei Reformatsky at the University of Kiev dis- covered that treatment of an a-haloester with zinc metal in the presence of an aldehyde or a ketone followed by hydrolysis in aqueous acid results in formation of a B-hydroxyester. This reaction is similar to a Grignard reaction in that a key inter- mediate is an organometallic compound, in this case, a zinc salt of an ester enolate anion. Grignard reagents, however, are so reactive that they undergo self-condensation with the ester. O [ZnBr]+ ОН Zn 1. PHCHO BRCH,COE. CH=COEL PHCHCH,COEt benzene 2. Н,О, НС Zinc salt of an enolate anion A B-hydroxyester (rácemic) Show how a Reformatsky reaction can be used to synthesize these compounds from an aldehyde or a ketone and an a-haloester. ОН (four stereoisomers: two racemic mixtures) as
In 1887, the Russian chemist Sergei Reformatsky at the University of Kiev dis- covered that treatment of an a-haloester with zinc metal in the presence of an aldehyde or a ketone followed by hydrolysis in aqueous acid results in formation of a B-hydroxyester. This reaction is similar to a Grignard reaction in that a key inter- mediate is an organometallic compound, in this case, a zinc salt of an ester enolate anion. Grignard reagents, however, are so reactive that they undergo self-condensation with the ester. O [ZnBr]+ ОН Zn 1. PHCHO BRCH,COE. CH=COEL PHCHCH,COEt benzene 2. Н,О, НС Zinc salt of an enolate anion A B-hydroxyester (rácemic) Show how a Reformatsky reaction can be used to synthesize these compounds from an aldehyde or a ketone and an a-haloester. ОН (four stereoisomers: two racemic mixtures) as
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter19: Enolate Anions And Enamines
Section: Chapter Questions
Problem 19.37P
Related questions
Question
![In 1887, the Russian chemist Sergei Reformatsky at the University of Kiev dis-
covered that treatment of an a-haloester with zinc metal in the presence of an
aldehyde or a ketone followed by hydrolysis in aqueous acid results in formation of
a B-hydroxyester. This reaction is similar to a Grignard reaction in that a key inter-
mediate is an organometallic compound, in this case, a zinc salt of an ester enolate anion.
Grignard reagents, however, are so reactive that they undergo self-condensation
with the ester.
O [ZnBr]+
ОН
Zn
1. PHCHO
BRCH,COE.
CH=COEL
PHCHCH,COEt
benzene
2. Н,О, НС
Zinc salt of an
enolate anion
A B-hydroxyester
(rácemic)
Show how a Reformatsky reaction can be used to synthesize these compounds from an
aldehyde or a ketone and an a-haloester.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F70c2953e-3acb-4fa9-9fb3-e4694e7622ce%2F1c543e2d-515e-4287-9eb7-82f7174ba08a%2Fysm0pcs.jpeg&w=3840&q=75)
Transcribed Image Text:In 1887, the Russian chemist Sergei Reformatsky at the University of Kiev dis-
covered that treatment of an a-haloester with zinc metal in the presence of an
aldehyde or a ketone followed by hydrolysis in aqueous acid results in formation of
a B-hydroxyester. This reaction is similar to a Grignard reaction in that a key inter-
mediate is an organometallic compound, in this case, a zinc salt of an ester enolate anion.
Grignard reagents, however, are so reactive that they undergo self-condensation
with the ester.
O [ZnBr]+
ОН
Zn
1. PHCHO
BRCH,COE.
CH=COEL
PHCHCH,COEt
benzene
2. Н,О, НС
Zinc salt of an
enolate anion
A B-hydroxyester
(rácemic)
Show how a Reformatsky reaction can be used to synthesize these compounds from an
aldehyde or a ketone and an a-haloester.
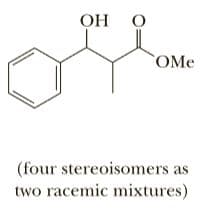
Transcribed Image Text:ОН
(four stereoisomers:
two racemic mixtures)
as
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT