In a certain chemical reaction Compound A combines with Compound B to produce Compound C (and no other products). Measurements were taken of the amounts of A and B present before and after a reaction that produced some C: Compound initial amount final amount A 5.5 g 0g B 1.5 g 1.1 g Calculate the theoretical yield of C. 5.9 g Round your answer to the nearest 0.1 g. Suppose the percent yield of C in this reaction was 50.%. Calculate the actual amount of C that was isolated at the end of the reaction. 2.95 g Round your answer to the nearest 0.1 g. olo
In a certain chemical reaction Compound A combines with Compound B to produce Compound C (and no other products). Measurements were taken of the amounts of A and B present before and after a reaction that produced some C: Compound initial amount final amount A 5.5 g 0g B 1.5 g 1.1 g Calculate the theoretical yield of C. 5.9 g Round your answer to the nearest 0.1 g. Suppose the percent yield of C in this reaction was 50.%. Calculate the actual amount of C that was isolated at the end of the reaction. 2.95 g Round your answer to the nearest 0.1 g. olo
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter4: Stoichiometry Of Chemical Reactions
Section: Chapter Questions
Problem 65E: Freon-12, CCl2F2, is prepared from CCl4 by reaction with HF. The other product of this reaction is...
Related questions
Question
Anser was confirmed inncorrect as attached can someone take a look and help me out please?
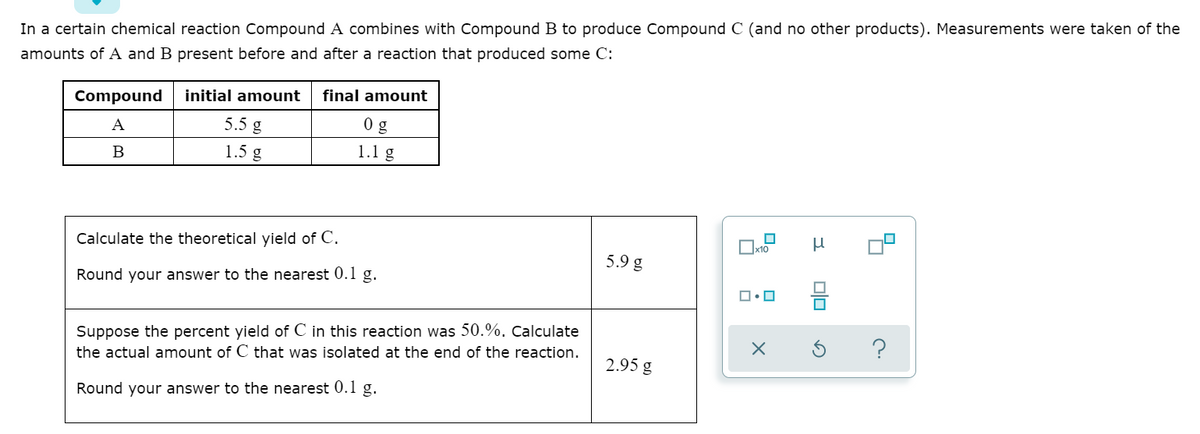
Transcribed Image Text:In a certain chemical reaction Compound A combines with Compound B to produce Compound C (and no other products). Measurements were taken of the
amounts of A and B present before and after a reaction that produced some C:
initial amount
final amount
Compound
A
5.5 g
B
1.5 g
1.1 g
Ox10
Calculate the theoretical yield of C.
5.9 g
Round your answer to the nearest 0.1 g.
Suppose the percent yield of C in this reaction was 50.%. Calculate
?
the actual amount of C that was isolated at the end of the reaction.
2.95 g
Round your answer to the nearest 0.1 g.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning