In an example Friedel-Crafts acylation, the electrophile is generated from an acid chloride (CH,CH,CH,COCI) and a Lewis acid (FeCl, ). Identify cach step of the mechanism and draw the resulting product from cach step. A. What is the first step of the mechanism? A curved arrow starts from a chlorine in FeCl, and points towards the chlorine in the acid chloride. A curved arrow starts from the bond between the carbonyl and chlorine in the acid chloride and points to the iron. A curved arrow starts from a chlorine in FeCl, and points towards the carbonyl. A curved arrow starts from the lone pair of the chlorine in the acid chloride and points to the iron. B. Add bonds, electron pairs and charges to show the product of the first step. Select Draw Rings More Erase Fe :a: Fe C. Which bond is broken in the second step of the mechanism? the bond between the carbonyl and the chlorine the bond between the carbonyl and the alkyl group the bond between the iron and the chlorine D. Draw the products of the second step of the mechanism. Be sure to add lone pairs and charges, where applicable. Select Rings More Erase Draw 0 000
In an example Friedel-Crafts acylation, the electrophile is generated from an acid chloride (CH,CH,CH,COCI) and a Lewis acid (FeCl, ). Identify cach step of the mechanism and draw the resulting product from cach step. A. What is the first step of the mechanism? A curved arrow starts from a chlorine in FeCl, and points towards the chlorine in the acid chloride. A curved arrow starts from the bond between the carbonyl and chlorine in the acid chloride and points to the iron. A curved arrow starts from a chlorine in FeCl, and points towards the carbonyl. A curved arrow starts from the lone pair of the chlorine in the acid chloride and points to the iron. B. Add bonds, electron pairs and charges to show the product of the first step. Select Draw Rings More Erase Fe :a: Fe C. Which bond is broken in the second step of the mechanism? the bond between the carbonyl and the chlorine the bond between the carbonyl and the alkyl group the bond between the iron and the chlorine D. Draw the products of the second step of the mechanism. Be sure to add lone pairs and charges, where applicable. Select Rings More Erase Draw 0 000
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
ChapterNW4: Nomenclature Worksheet 4: Carbonyl Compounds
Section: Chapter Questions
Problem 15CTQ
Related questions
Question
parts C and D. Thank you
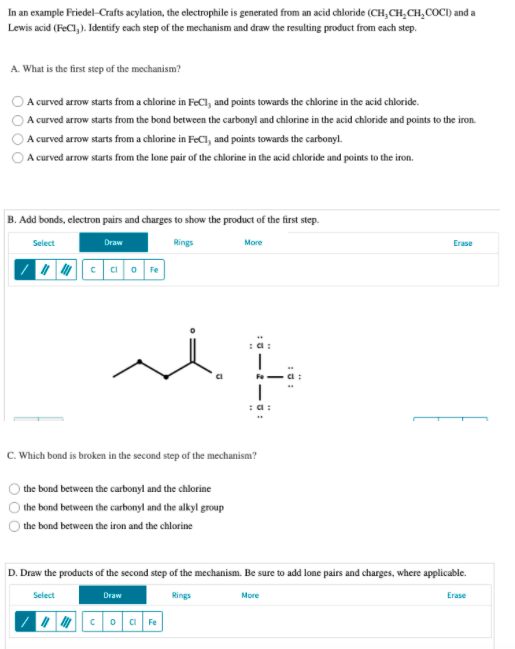
Transcribed Image Text:In an example Friedel-Crafts acylation, the electrophile is generated from an acid chloride (CH,CH,CH,COCI) and a
Lewis acid (FeCl, ). Identify cach step of the mechanism and draw the resulting product from cach step.
A. What is the first step of the mechanism?
A curved arrow starts from a chlorine in FeCl, and points towards the chlorine in the acid chloride.
A curved arrow starts from the bond between the carbonyl and chlorine in the acid chloride and points to the iron.
A curved arrow starts from a chlorine in FeCl, and points towards the carbonyl.
A curved arrow starts from the lone pair of the chlorine in the acid chloride and points to the iron.
B. Add bonds, electron pairs and charges to show the product of the first step.
Select
Draw
Rings
More
Erase
Fe
:a:
Fe
C. Which bond is broken in the second step of the mechanism?
the bond between the carbonyl and the chlorine
the bond between the carbonyl and the alkyl group
the bond between the iron and the chlorine
D. Draw the products of the second step of the mechanism. Be sure to add lone pairs and charges, where applicable.
Select
Draw
Rings
More
Erase
I c o a Fe
0 000
Expert Solution
Step 1
Friedel Craft acylation :-
In this reaction generation of electrophilic acyl group from acyl chloride react with anhydrous FeCl3 or AlCl3 . Now the electrophilic acyl group attack on benzene ring or its derivatives to form final product acyl benzene.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning