In an experiment to determine the content of acetylsalicylic acid in an aspirin tablet, the result showed that the calculated mass of acetylsalicylic acid was much higher than the indicated amount in the packaging. Identify two among the following situations that can most likely be the cause. (You can use the formula shown as guide). amount of base amount of unreacted base/ added amount titrated mass analyte = (Vbase x Mpase) – (Vacia X Macia x mole base mole acid mole analyte x molar massanalyte mole base =amount of base that reacted The unreacted base was overtitrated during back titration. After grinding, some of the pulverized tablet was not transferred to the flask. The mass of the tablet recorded included that of the packaging. The tablet contained other acids that can be neutralized by the base. A mole ratio of 1:1 (acetylsalicylic acid:NaOH) was used instead of 1:2. The aspirin was not completely hydrolyzed. The molar mass of salicylic acid was used instead of acetylsalicylic acid in the calculations.
In an experiment to determine the content of acetylsalicylic acid in an aspirin tablet, the result showed that the calculated mass of acetylsalicylic acid was much higher than the indicated amount in the packaging. Identify two among the following situations that can most likely be the cause. (You can use the formula shown as guide). amount of base amount of unreacted base/ added amount titrated mass analyte = (Vbase x Mpase) – (Vacia X Macia x mole base mole acid mole analyte x molar massanalyte mole base =amount of base that reacted The unreacted base was overtitrated during back titration. After grinding, some of the pulverized tablet was not transferred to the flask. The mass of the tablet recorded included that of the packaging. The tablet contained other acids that can be neutralized by the base. A mole ratio of 1:1 (acetylsalicylic acid:NaOH) was used instead of 1:2. The aspirin was not completely hydrolyzed. The molar mass of salicylic acid was used instead of acetylsalicylic acid in the calculations.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter4: Types Of Chemical Reactions And Solution Stoichiometry
Section: Chapter Questions
Problem 47E
Related questions
Question
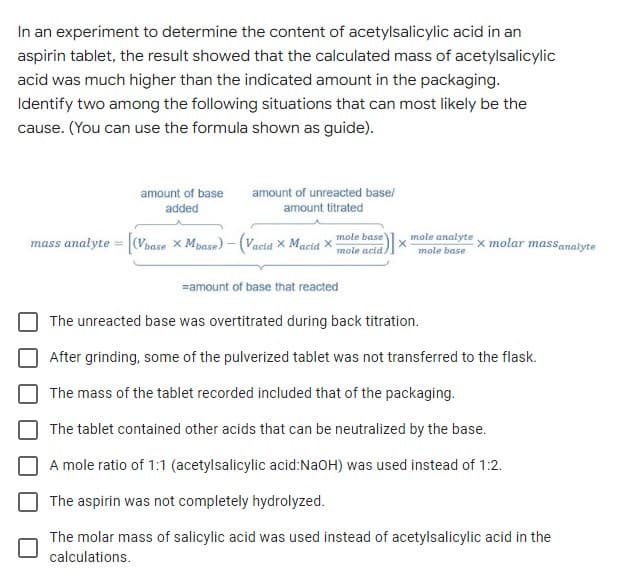
Transcribed Image Text:In an experiment to determine the content of acetylsalicylic acid in an
aspirin tablet, the result showed that the calculated mass of acetylsalicylic
acid was much higher than the indicated amount in the packaging.
Identify two among the following situations that can most likely be the
cause. (You can use the formula shown as guide).
amount of base
amount of unreacted base/
added
amount titrated
mass analyte = (Vbase x Mpase) -(Vacid X Macid X
mole base
mole analyte
x molar massanalyte
mole acid.
mole base
=amount of base that reacted
The unreacted base was overtitrated during back titration.
After grinding, some of the pulverized tablet was not transferred to the flask.
The mass of the tablet recorded included that of the packaging.
The tablet contained other acids that can be neutralized by the base.
A mole ratio of 1:1 (acetylsalicylic acid:NaOH) was used instead of 1:2.
The aspirin was not completely hydrolyzed.
The molar mass of salicylic acid was used instead of acetylsalicylic acid in the
calculations.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning