In mass spectrometry, there are patterns in the molecular ion region due to isotopic abundances. Identify the pattern that would correspond to a molecule containing one bromine and one chlorine. || T. A)I M-2 Me Me2 Me4 B) II II IV C) II M2 Me4 M Me4 D) IV E) V Me2 Me4
In mass spectrometry, there are patterns in the molecular ion region due to isotopic abundances. Identify the pattern that would correspond to a molecule containing one bromine and one chlorine. || T. A)I M-2 Me Me2 Me4 B) II II IV C) II M2 Me4 M Me4 D) IV E) V Me2 Me4
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter11: Atomic Mass Spectrometry
Section: Chapter Questions
Problem 11.2QAP
Related questions
Question
100%
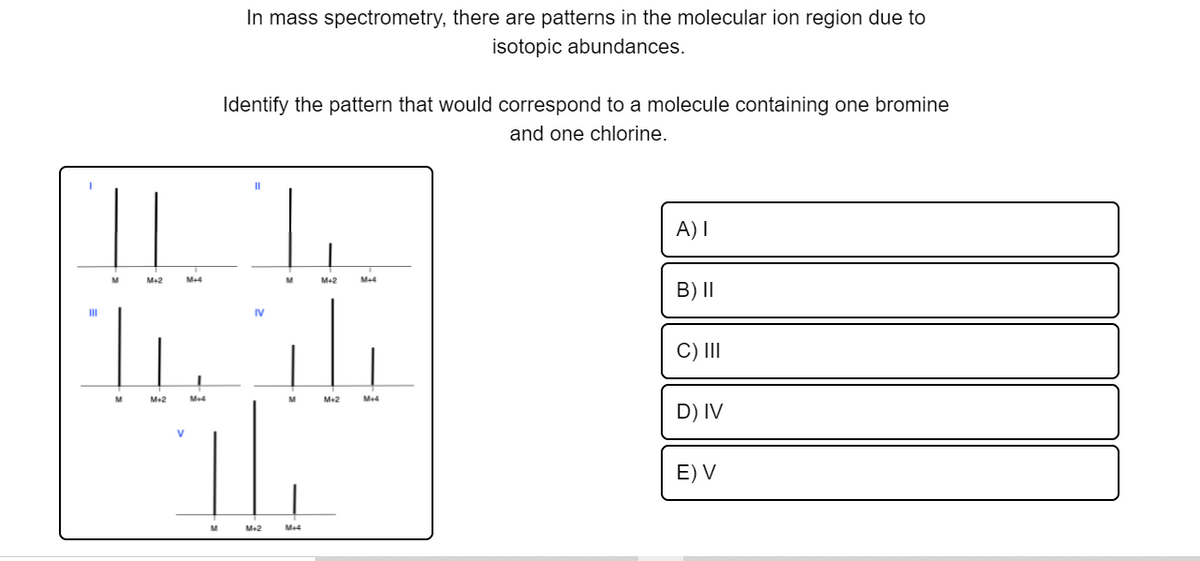
Transcribed Image Text:In mass spectrometry, there are patterns in the molecular ion region due to
isotopic abundances.
Identify the pattern that would correspond to a molecule containing one bromine
and one chlorine.
|| |.
A) I
M+2
M+4
M
Me2
B) II
II
IV
C) II
M+2
Me4
M-2
Me4
D) IV
E) V
M+2
Me4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning