In the chemical synthesis of DNA and RNA, hydroxyl groups are normally converted to triphenylmethyl (trityl) ethers to protect the hydroxyl group from reaction with other reagents. neutralized by the tertiary amine tertiary amine RCH,OH Ph,CCI RCH,OCP., HCI Triphenylmethyl chloride (Trityl chloride) A triphenylmethyl ether (A trityl ether) Triphenylmethyl ethers are stable to aqueous base but are rapidly cleaved in aqueous acid. RCH,OCPH3 + H2O RCH2OH + Ph3COH
In the chemical synthesis of DNA and RNA, hydroxyl groups are normally converted to triphenylmethyl (trityl) ethers to protect the hydroxyl group from reaction with other reagents. neutralized by the tertiary amine tertiary amine RCH,OH Ph,CCI RCH,OCP., HCI Triphenylmethyl chloride (Trityl chloride) A triphenylmethyl ether (A trityl ether) Triphenylmethyl ethers are stable to aqueous base but are rapidly cleaved in aqueous acid. RCH,OCPH3 + H2O RCH2OH + Ph3COH
Chapter22: Carbonyl Alpha-substitution Reactions
Section22.SE: Something Extra
Problem 63AP: As far back as the 16th century, South American Incas chewed the leaves of the coca bush,...
Related questions
Question
How might the structure of the triphenylmethyl group be modified to increase or decrease its acid sensitivity?
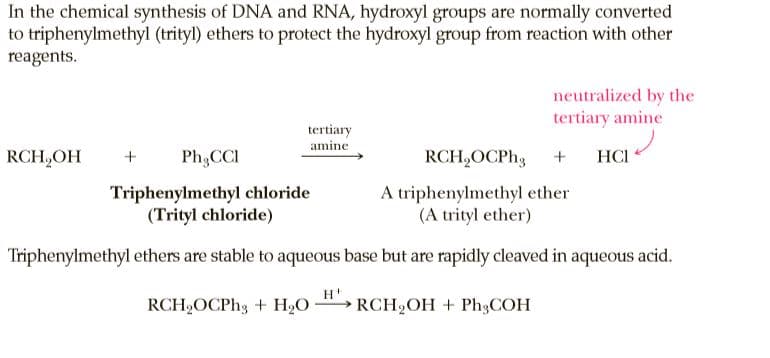
Transcribed Image Text:In the chemical synthesis of DNA and RNA, hydroxyl groups are normally converted
to triphenylmethyl (trityl) ethers to protect the hydroxyl group from reaction with other
reagents.
neutralized by the
tertiary amine
tertiary
amine
RCH,OH
Ph,CCI
RCH,OCP.,
HCI
Triphenylmethyl chloride
(Trityl chloride)
A triphenylmethyl ether
(A trityl ether)
Triphenylmethyl ethers are stable to aqueous base but are rapidly cleaved in aqueous acid.
RCH,OCPH3 + H2O
RCH2OH + Ph3COH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole