Explain why all classes of low molecular weight amines are water soluble. O Low molecular weight amines are basic enough to form alkylammonium cations in water. Once such cation is formed, the solubility of amine drastically increases. The polarity of low molecular weight amines is much greater than that of water, thus allowing the molecules of these amines to be freely distributed among molecules of water. O Low molecular weight amines are gases at STP and gases usually have high solubility in water. O All amines can form hydrogen bonds with water. Low molecular weight amines have small aliphatic portions, thus the hydrogen bonds to water are strong enough to allow the amines to
Explain why all classes of low molecular weight amines are water soluble. O Low molecular weight amines are basic enough to form alkylammonium cations in water. Once such cation is formed, the solubility of amine drastically increases. The polarity of low molecular weight amines is much greater than that of water, thus allowing the molecules of these amines to be freely distributed among molecules of water. O Low molecular weight amines are gases at STP and gases usually have high solubility in water. O All amines can form hydrogen bonds with water. Low molecular weight amines have small aliphatic portions, thus the hydrogen bonds to water are strong enough to allow the amines to
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter23: Amines
Section: Chapter Questions
Problem 23.19P
Related questions
Question
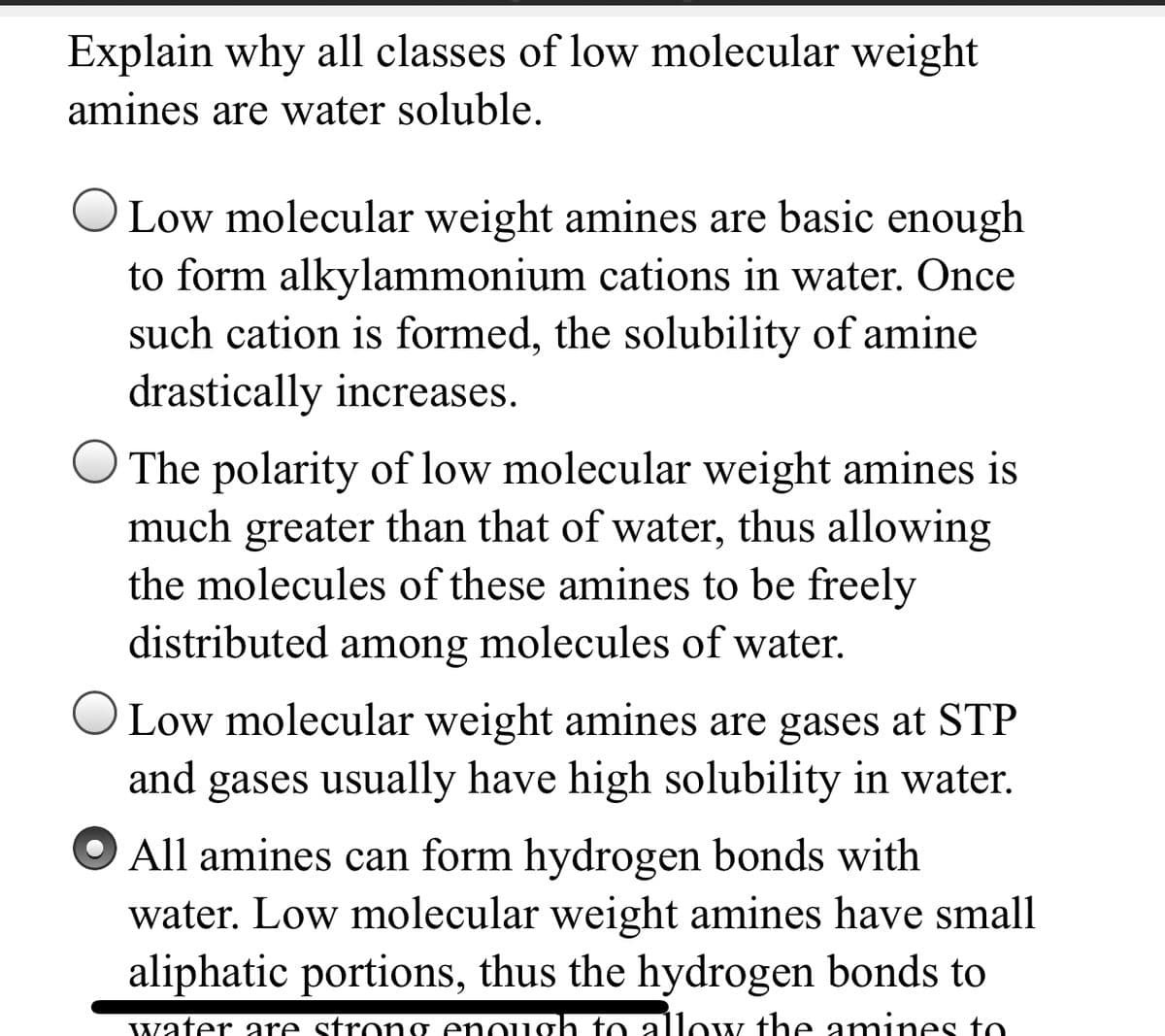
Transcribed Image Text:Explain why all classes of low molecular weight
amines are water soluble.
O Low molecular weight amines are basic enough
to form alkylammonium cations in water. Once
such cation is formed, the solubility of amine
drastically increases.
O The polarity of low molecular weight amines is
much greater than that of water, thus allowing
the molecules of these amines to be freely
distributed among molecules of water.
O Low molecular weight amines are gases at STP
and gases usually have high solubility in water.
All amines can form hydrogen bonds with
water. Low molecular weight amines have small
aliphatic portions, thus the hydrogen bonds to
water are strong enough to allow the amines to
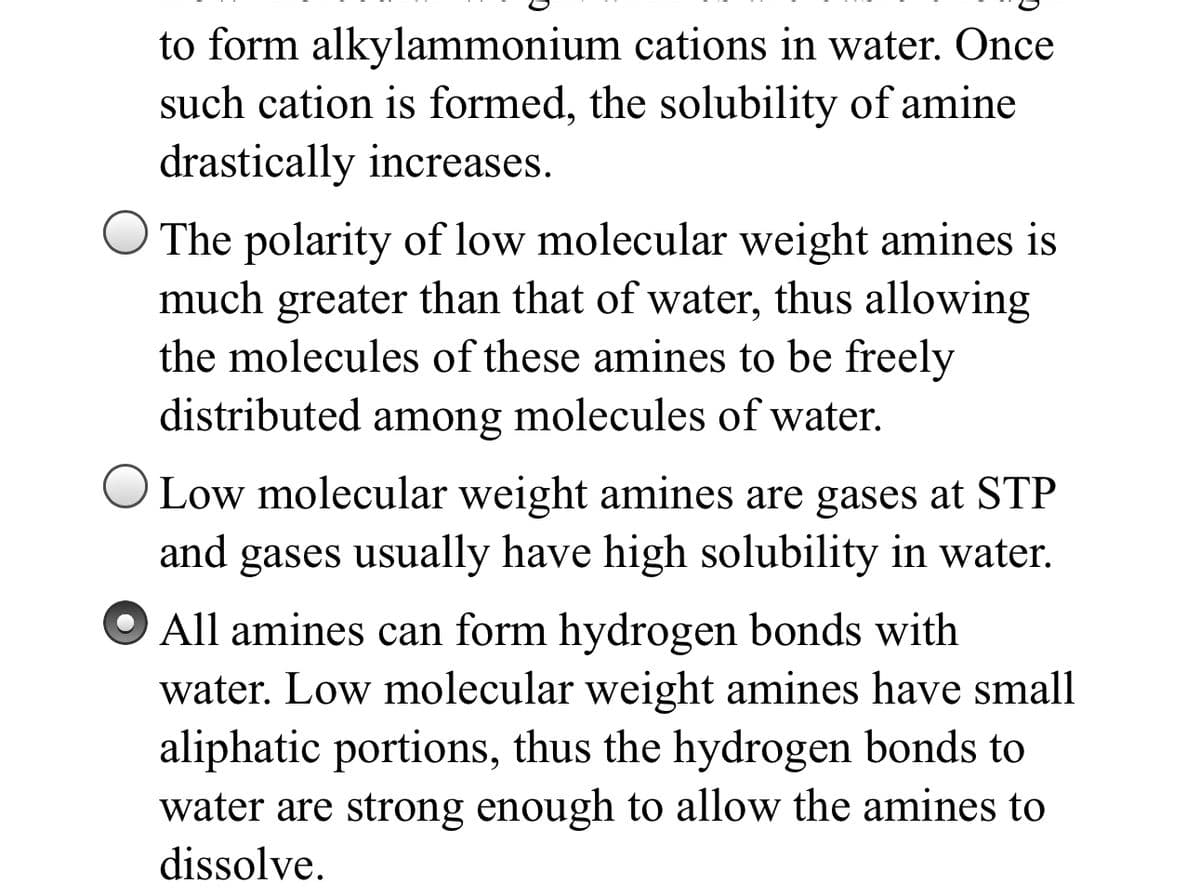
Transcribed Image Text:to form alkylammonium cations in water. Once
such cation is formed, the solubility of amine
drastically increases.
O The polarity of low molecular weight amines is
much greater than that of water, thus allowing
the molecules of these amines to be freely
distributed among molecules of water.
OLow molecular weight amines are gases at STP
and
gases usually have high solubility in water.
All amines can form hydrogen bonds with
water. Low molecular weight amines have small
aliphatic portions, thus the hydrogen bonds to
water are strong enough to allow the amines to
dissolve.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole