In the following table are reported (at 15.7°C) the values of the ratio, PV/RT, for one mole of air, at different pressures. When gas behaves as a perfect gas this ratio is equal to the unit. At 100 bar, chose the correct statement: Pressure (bar) 1 C. PV RT e. 100 1 0.9824 150 0.9976 200 a. The volume occupied at constant T and P is greater than for the ideal gas b. There are repulsive interactions between the air molecules. The gas is more compressible than ideal gas d. We can put less than one mole of air in the same volume, compared to ideal gas. The gas is still ideal 1.0265
In the following table are reported (at 15.7°C) the values of the ratio, PV/RT, for one mole of air, at different pressures. When gas behaves as a perfect gas this ratio is equal to the unit. At 100 bar, chose the correct statement: Pressure (bar) 1 C. PV RT e. 100 1 0.9824 150 0.9976 200 a. The volume occupied at constant T and P is greater than for the ideal gas b. There are repulsive interactions between the air molecules. The gas is more compressible than ideal gas d. We can put less than one mole of air in the same volume, compared to ideal gas. The gas is still ideal 1.0265
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter4: Introduction To Gases
Section: Chapter Questions
Problem 67E: The compression ratio in an automobile engine is the ratio of the gas pressure at the end of the...
Related questions
Question
40
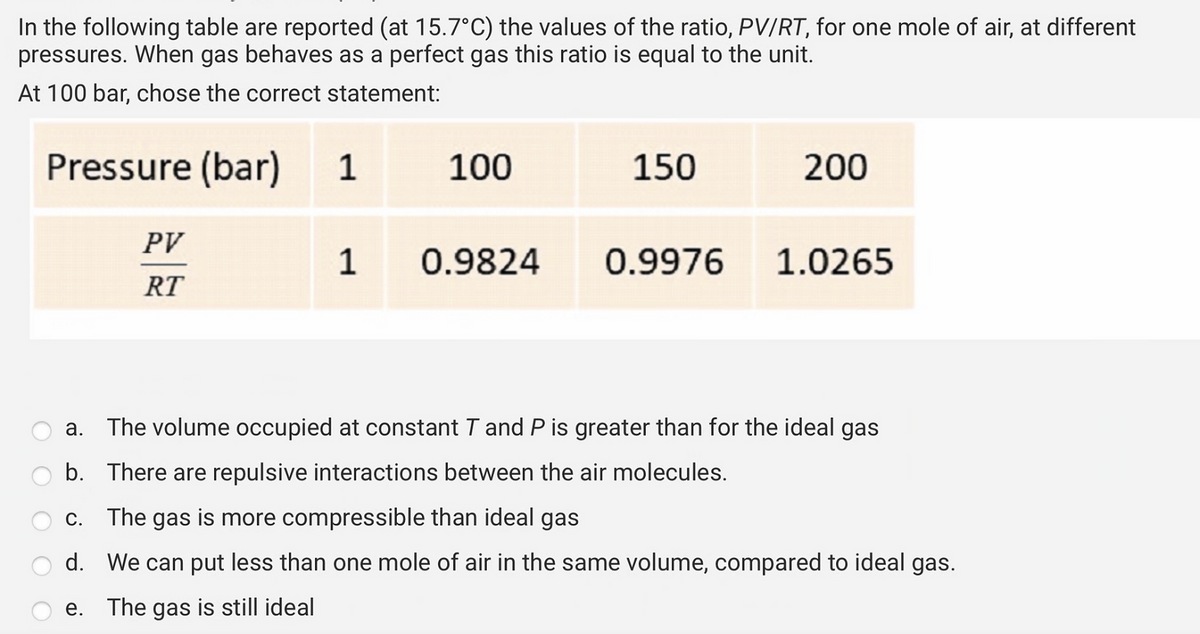
Transcribed Image Text:In the following table are reported (at 15.7°C) the values of the ratio, PV/RT, for one mole of air, at different
pressures. When gas behaves as a perfect gas this ratio is equal to the unit.
At 100 bar, chose the correct statement:
Pressure (bar) 1
PV
RT
1
100
0.9824
150
0.9976
200
1.0265
a. The volume occupied at constant T and P is greater than for the ideal gas
b. There are repulsive interactions between the air molecules.
c. The gas is more compressible than ideal gas
d.
We can put less than one mole of air in the same volume, compared to ideal gas.
e. The gas is still ideal
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning