In the free radical chlorination of isobutane, what is the % yield of product X and Y, respectively? H CH3 CH3 + HC- H - | A. X=64%, Y=36% B. X=36%, Y=64% C. X=60%, Y=40% D. X=40%, Y=60% E. X=trace (<1%), Y=>99% F. X=28%, Y=72%
In the free radical chlorination of isobutane, what is the % yield of product X and Y, respectively? H CH3 CH3 + HC- H - | A. X=64%, Y=36% B. X=36%, Y=64% C. X=60%, Y=40% D. X=40%, Y=60% E. X=trace (<1%), Y=>99% F. X=28%, Y=72%
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter15: Radical Reactions
Section: Chapter Questions
Problem 10E
Related questions
Question
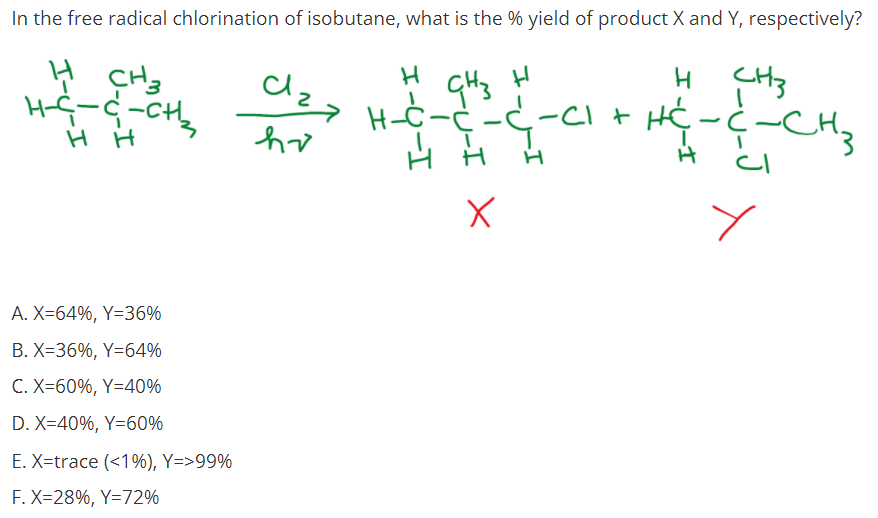
Transcribed Image Text:In the free radical chlorination of isobutane, what is the % yield of product X and Y, respectively?
dzz H-Ċ-C
H CH3
-CI t HC-ċ-CH2
H CH3
H H
A. X=64%, Y=36%
B. X=36%, Y=64%
C. X=60%, Y=40%
D. X=40%, Y=60%
E. X=trace (<1%), Y=>99%
F. X=28%, Y=72%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax