in the laboratory you dissolve 22.5 g of aluminum iodide in a volumetric flask and add water to total volume of 500 mL. What is the molarity of the solution? M. What is the concentration of the aluminum cation? M. What is the concentration of the iodide anion? Submit Answer M. Retry Entire Group 5 more group attempts remaining
in the laboratory you dissolve 22.5 g of aluminum iodide in a volumetric flask and add water to total volume of 500 mL. What is the molarity of the solution? M. What is the concentration of the aluminum cation? M. What is the concentration of the iodide anion? Submit Answer M. Retry Entire Group 5 more group attempts remaining
Living By Chemistry: First Edition Textbook
1st Edition
ISBN:9781559539418
Author:Angelica Stacy
Publisher:Angelica Stacy
ChapterU4: Toxins: Stoichiometry, Solution Chemistry, And Acids And Bases
SectionU4.14: Drop In: Molecular Views
Problem 5E
Related questions
Question
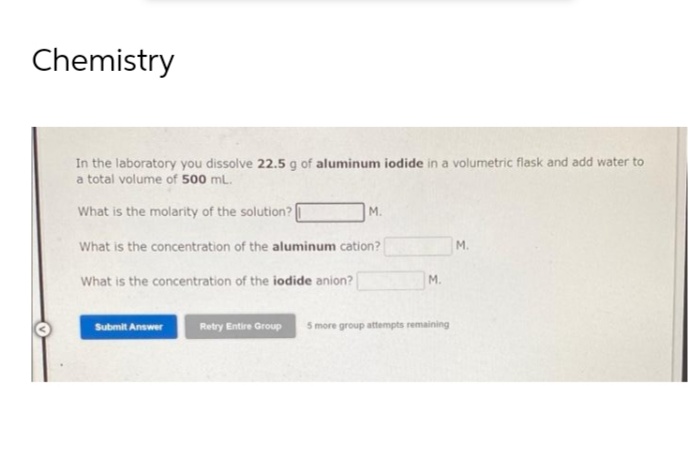
Transcribed Image Text:Chemistry
In the laboratory you dissolve 22.5 g of aluminum iodide in a volumetric flask and add water to
a total volume of 500 mL.
What is the molarity of the solution?
M.
What is the concentration of the aluminum cation?
M.
What is the concentration of the iodide anion?
Submit Answer
M.
Retry Entire Group 5 more group attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
